Ranitidine: instructions for use. The drug "Ranitidine": indications for use, composition and properties Ranitidine indications for use
Description
Beige-yellow color, round, biconvex, film-coated tablets with the inscription "IL" on one side.
Structure
Each film-coated tablet contains:
active substance: ranitidine hydrochloride in an amount equivalent to 150 mg of ranitidine.
excipients:microcrystalline cellulose, croscarmellose sodium, anhydrous colloidal silicon dioxide, talc, magnesium stearate, hypromellose E15, castor oil, titanium dioxide E171, iron oxide yellow.
Pharmacotherapeutic group
Antiulcer drugs and drugs used for gastroesophageal reflux disease (GERD). Blockers of histamine H2-receptors. The codeATX: A02BA02.
Pharmacological properties
Pharmacodynamics
Ranitidine is a competitive antagonist of histamine H2 receptors. Suppresses basal and stimulated secretion of hydrochloric acid, reduces the volume of gastric juice, the content of hydrochloric acid and pepsin in it. Taking one dose of 150 mg of the drug causes a decrease in the production of hydrochloric acid lasting up to 12 hours.
Pharmacokinetics
The absolute bioavailability of ranitidine is 50-60%. After taking the drug orally at a dose of 150 mg, the maximum plasma concentrations (usually in the range of 300-550 ng / ml) are observed after 2-3 hours. In doses up to 300 mg, the plasma ranitidine concentrations increase in proportion to the dose increase.
Plasma protein binding is about 15%, the volume of distribution is in the range from 96 to 142 liters.
It is excreted mainly by the kidneys by tubular secretion. The half-life is 2-3 hours. After oral administration of 3H-ranitidine at a dose of 150 mg, 60-70% of the drug was excreted in the urine and 26% in the faeces, and 35% of the dose taken was excreted in the urine unchanged. Metabolism of ranitidine does not differ between parenteral administration and oral administration and proceeds with the formation of small amounts of N-oxide (6%), S-oxide (2%), desmethylranitidine (2%) and an analog of furoic acid (1-2%).
Children
Limited pharmacokinetic data indicate that there are no significant differences in half-life (range for children 3 years and older: 1.7-2.2 h) and plasma clearance (range for children 3 years and older: 9-22 ml / min / kg) in children and healthy adults receiving oral ranitidine, when the dose is adjusted depending on body weight.
Patients over 50
In patients over 50 years of age, the elimination half-life increases (3-4 hours), plasma clearance decreases in accordance with the age-related decline in renal function. However, systemic exposure and uptake are 50% higher. This difference exceeds the effect of decreasing renal function and indicates an increased bioavailability in elderly patients.
Indications for use
Adults
Ulcers duodenum and benign stomach ulcers, including those associated with the use of non-steroidal anti-inflammatory drugs (NSAIDs).
Prevention of duodenal ulcers caused by NSAIDs (including aspirin), especially in patients with a history of peptic ulcer.
Treatment of duodenal ulcer associated with Helicobacter pylori infection. Postoperative ulcers.
Reflux esophagitis, also for long-term use. Relief of symptoms of gastroesophageal reflux disease. Zollinger-Ellison syndrome.
Chronic episodic dyspepsia, characterized by epigastric or chest pain that is associated with eating or disturbing sleep, but not related to the above conditions.
Prevention of gastrointestinal bleeding from stress ulcers in critically ill patients.
Prevention of recurrent bleeding in patients with bleeding peptic ulcers. Before anesthesia in patients with a high risk of aspiration of acidic stomach contents (Mendelssohn's syndrome), especially in women in labor.
Children over 12 years old
Short-term treatment of peptic ulcers.
Treating gastroesophageal reflux, including reflux esophagitis and relieving symptoms of gastroesophageal reflux disease.
Method of administration and dosage
Is taken orally, regardless of food intake.
Adults (including elderly patients)
The usual dose is 150 mg twice a day (morning and evening).
Duodenal ulcers and benign stomach ulcers
The usual recommended dose is 150 mg twice daily or 300 mg once daily (overnight). In most cases, healing of duodenal ulcers, benign stomach ulcers, and postoperative ulcers occurs within 4 weeks. Ulcers unharmed during this period usually heal with continued treatment for the next 4 weeks.
Ulcers associated with taking NSAIDs.
An 8 week course of treatment may be required.
Prevention of duodenal ulcers caused by NSAIDs
150 mg twice a day, concurrently with NSAID therapy.
In the treatment of duodenal ulcers, a dose of 300 mg twice a day for 4 weeks is more effective than dosage regimens of 150 mg 2 times a day or 300 mg 1 time per day (at night). Dose increases do not increase frequency side effects.
Duodenal ulcers associated with infectionHelicobacter pylori. A dose of 300 mg at night or 150 mg 2 times a day is used in combination with amoxicillin 750 mg 3 times a day and metronidazole 500 mg 3 times a day for 2 weeks. Treatment with ranitidine should be continued for another 2 weeks. This regimen of therapy significantly reduces the frequency of recurrence of duodenal ulcers. Maintenance therapy with a reduced dose of 150 mg at bedtime is recommended in patients who have benefited from short-term treatment, especially in patients with a history of recurrent ulcers.
Gastroesophageal reflux disease
To relieve the symptoms of gastroesophageal reflux disease, it is recommended to take ranitidine 150 mg twice daily for 2 weeks. In case of insufficient effectiveness, treatment can be continued at the same dose for the next 2 weeks.
For the treatment of reflux esophagitis, a dose of 150 mg twice a day or 300 mg once a day (at night) is recommended for 8 weeks, if necessary, the course of treatment can be extended to 12 weeks. With moderate and severe reflux esophagitis, the dose can be increased to 150 mg 4 times a day with a duration of treatment up to 12 weeks.
SyndromeZollinger - Ellison
The starting dose is 150 mg three times a day. The dose can be increased if necessary. Doses up to 6 g per day were well tolerated.
Chronic episodic dyspepsia
Prevention of bleeding from stress ulcers in critically ill patients and preventionrecurrent bleeding from peptic ulcers
After the patient is able to eat, the injections of ranitidine can be replaced by taking tablets at a dose of 150 mg twice a day.
Prevention of Mendelssohn's syndrome
During labor, women in labor are advised to administer ranitidine 150 mg by mouth every 6 hours. If a woman in labor requires general anesthesia, water-soluble antacids (for example, sodium citrate) should be additionally used in front of her along with ranitidine. The usual precautions must be taken to prevent aspiration of gastric juice.
Children over 12 years old
The recommended daily dose for the treatment of gastroesophageal reflux disease is 5-10 mg / kg / body weight, divided into two doses. Maximum daily dose 600 mg ( maximum dose can be used in children or adolescents with severe symptoms). Application for renal failure
In patients with renal failure (creatinine clearance less than 50 ml / min) cumulation and an increase in the plasma concentration of ranitidine are noted. In such patients, the daily dose should be 150 mg (at night) for 4-8 weeks. The same dose is recommended for maintenance therapy. If the ulcer does not heal, treatment should be continued at a dose of 150 mg twice a day, and then, if necessary, switch to maintenance therapy 150 mg per day (at night).
If the drug intake has been missed, it is necessary to take it as soon as the patient remembered about it, if it is not yet time to take the next dose. Do not take a double dose to make up for the missed dose.
Contraindications
Hypersensitivity to the active substance or any of the components of the drug. Before starting therapy with ranitidine, it is necessary to exclude malignant neoplasms.
Precautions
Treatment with the drug can mask the symptoms of gastric carcinoma, therefore, before starting treatment, the presence of malignant neoplasms in the stomach should be excluded. Ranitidine is mainly excreted through the kidneys, therefore, in patients with renal insufficiency, its plasma level is increased and the dose should be adjusted. It is assumed that ranitidine can cause acute porphyric attacks in patients with acute porphyria, therefore, its use in patients with acute porphyria (including a history) should be avoided.
It is necessary to regularly monitor patients (especially the elderly and with a history of peptic ulcers of the stomach and / or duodenum) who take ranitidine together with non-steroidal anti-inflammatory drugs.
Post-marketing data indicate cases of reversible confusion, depression and hallucinations, which were most common in critically ill and elderly patients.
In elderly patients, persons with chronic diseases lungs, diabetes mellitus or individuals with weakened immunity have an increased propensity to develop community-acquired pneumonia.
Smoking can reduce the effectiveness of the drug.
Treatment with the drug is gradually canceled due to the risk of developing the "rebound" syndrome in case of abrupt withdrawal.
Due to the presence of lactose in the preparation, its use is contraindicated in patients with rare congenital galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption.
Pregnancy and lactation
Ranitidine has the ability to cross the placenta, however, when therapeutic doses of the drug are prescribed to women in labor during labor or before by caesarean section no adverse effects on labor and development of the newborn were observed. The drug is excreted in breast milk. Like other medicines, ranitidine should be given to nursing mothers and pregnant women only if absolutely necessary.
Use by children
Safety and effectiveness in children has not been established.
Elderly use
The rate of ulcer treatment and the frequency of side effects in the elderly (65-82 years) does not differ from the rate and frequency in younger age groups.
Influence on the ability to drive vehicles and mechanisms
The drug can cause dizziness, drowsiness, hallucinations, impaired accommodation, and, therefore, affect the reaction rate when administered vehicles and other potentially dangerous species activities requiring increased concentration of attention and speed of psychomotor reactions.
Adverse reactions
Adverse reactions are presented in accordance with the classification of organ systems and frequency of occurrence: very often (≥ 1/10), often (≥ 1/100 to
From the blood system: very rarely - leukopenia, granulocytopenia, thrombocytopenia (usually reversible), agranulocytosis or pancytopenia, sometimes with bone marrow hypoplasia and aplastic anemia, neutropenia; very rarely - immune hemolytic anemia.
From the side immune system: rarely - hypersensitivity reactions: urticaria, angioedema, fever, bronchospasm, hypotension, chest pain; very rarely - anaphylactic shock.
From the side of the psyche: very rarely - increased fatigue, reversible confusion, drowsiness, insomnia, depression, hallucinations, tinnitus, irritability, disorientation. These manifestations are observed mainly in seriously ill or elderly patients.
From the side nervous system: very rarely - headache, dizziness and reversible involuntary movement disorders.
On the part of the organ of vision: fuzziness visual perception, violation of accommodation.
On the part of the cardiovascular system: very rarely - vasculitis, arrhythmias such as bradycardia, tachycardia, asystole, AV block, extrasystole.
From the side digestive system: infrequently - abdominal pain, nausea, vomiting, constipation; very rarely - acute pancreatitis, diarrhea.
From the hepatobilyar systems: rarely - transient reversible changes in liver function indicators; very rarely - hepatocellular, cholestatic or mixed hepatitis, jaundice.
On the part of the skin and subcutaneous tissue: rarely - skin rash, very rarely - erythema multiforme, alopecia.
From the side of the musculoskeletal system: very rarely - arthralgia, myalgia.
From the urinary system: rarely - an increase in plasma creatinine; very rarely - acute interstitial nephritis.
On the part of the reproductive system: very rarely - galactorrhea, gynecomastia, decreased potency and / or libido.
Overdose
Symptoms: increased side effects.
Treatment: Removal of unabsorbed drug from gastrointestinal tract, clinical observation. If necessary, symptomatic and supportive therapy is performed. The drug can be removed by hemodialysis.
Interaction with other medicinal products
Ranitidine may interfere with the absorption, metabolism, or renal excretion of other drugs. Altered pharmacokinetics may require dosage adjustments of these drugs or discontinuation of treatment.
Interactions can be triggered by a variety of mechanisms, including:
Suppression of mixed-function oxygenase systems associated with cytochrome P-450.
There are reports of an increase in prothrombin time during the use of anticoagulants - coumarin derivatives, for example, warfarin. Given the narrow therapeutic index, prothrombin time should be carefully monitored during treatment with ranitidine.
Competition in the process of renal tubular secretion:
Since ranitidine is partially secreted by the cationic transport system of the kidneys, this can affect the clearance of other drugs eliminated in this way. High doses of ranitidine (in the treatment of Zollinger-Ellison syndrome) can reduce the excretion of procanamide and N-acetylprocainamide, increasing the concentration of these drugs in plasma.
PH change in the stomach:
The bioavailability of some drugs may be altered. This can lead to an increase (eg, triazolam, midazolam, glipizide) or a decrease in their absorption (eg, ketoconazole, atazanavir, delaviridine, gefitinib).
On prescription.
Registrant
CJSC Maxpharma Baltic,
st. Saltoniskiu 29/3, LT 08105
Vilnius, Republic of Lithuania.
Tel. +370 5 273 08 93.
Manufacturer
Intas Pharmaceuticals Ltd.,
2nd Floor, Chinubhai Center
Off. Nehru Bridge, Ashram Road
Ahmedabad-380 009, India.
Packed
Private production and trade unitary enterprise "Stimenergy"
Legal address: st. Engineering, 7/2, room 4, Minsk, Republic of Belarus.
Factory address: st. Sosnovy Bor, 4, Minsk, Republic of Belarus.
The page provides instructions for use Ranitidine... It is available in various dosage forms of the drug (tablets 150 mg and 300 mg), and also has a number of analogues. This annotation has been checked by specialists. Leave your feedback on the use of Ranitidine, which will help other site visitors. The drug is used for various diseases (stomach and duodenal ulcers, heartburn, reflux). The tool has a number of side effects and interactions with other substances. Doses of the drug differ for adults and children. There are restrictions on the use of the medication during pregnancy and during lactation. Ranitidine treatment can only be prescribed by a qualified doctor. The duration of therapy can vary and depends on the specific disease.
Instructions for use and reception scheme
Ranitidine is taken regardless of food intake, without chewing, with a small amount of liquid.
Peptic ulcer stomach and duodenum 12. For the treatment of exacerbations, 150 mg are prescribed 2 times a day (morning and evening) or 300 mg at night. If necessary, 300 mg 2 times a day. The duration of the course of treatment is 4-8 weeks. For the prevention of exacerbations, 150 mg is prescribed at night, for smoking patients - 300 mg at night.
Ulcers associated with the use of non-steroidal anti-inflammatory drugs (NSAIDs). Assign 150 mg 2 times a day or 300 mg at night for 8-12 weeks. Prevention of ulceration when taking NSAIDs - 150 mg 2 times a day.
Postoperative and stress ulcers. Assign 150 mg 2 times a day for 4-8 weeks.
Erosive reflux esophagitis. Assign 150 mg 2 times a day or 300 mg at night. If necessary, the dose can be increased to 150 mg 4 times a day. The course of treatment is 8-12 weeks. Long-term preventive therapy - 150 mg 2 times a day.
Zollinger-Ellison Syndrome. The initial dose is 150 mg 3 times a day, if necessary, the dose can be increased.
Prevention of recurrent bleeding. 150 mg 2 times a day.
Prevention of the development of Mendelssohn's syndrome. Prescribe a dose of 150 mg 2 hours before anesthesia, and preferably 150 mg the night before.
In the presence of concomitant liver dysfunction, dose reduction may be required.
For patients with renal insufficiency with CC less than 50 ml / min, the recommended dose is 150 mg per day.
Release forms
Film-coated tablets 150 mg and 300 mg.
Ranitidine - blocker of histamine H2-receptors. Reduces basal and stimulated secretion of hydrochloric acid caused by irritation of baroreceptors, food stress, the action of hormones and biogenic stimulants (gastrin, histamine, pentagastrin). Ranitidine reduces the volume of gastric juice and the content of hydrochloric acid in it, increases the pH of the stomach contents, which leads to a decrease in pepsin activity. After oral administration in therapeutic doses, it does not affect prolactin levels. Inhibits microsomal enzymes.
The duration of action after a single dose is up to 12 hours.
Pharmacokinetics
It is quickly absorbed, food intake does not affect the degree of absorption. Upon oral administration, the bioavailability of ranitidine is approximately 50%. Plasma protein binding does not exceed 15%. It is slightly metabolized in the liver to form desmethylranitidine and ranitidine S-oxide. It is excreted mainly in the urine (60-70%, unchanged - 35%), a small amount in the feces. Poorly penetrates the blood-brain barrier. Penetrates the placenta. It is excreted in breast milk (the concentration in breast milk in women during lactation is higher than in plasma).
Indications
- treatment and prevention of exacerbations of gastric ulcer and duodenal ulcer;
- ulcers of the stomach and duodenum associated with taking NSAIDs;
- reflux esophagitis, erosive esophagitis;
- zollinger-Ellison syndrome;
- treatment and prevention of postoperative, "stress" ulcers of the upper gastrointestinal tract;
- prevention of recurrence of bleeding from the upper gastrointestinal tract;
- prevention of aspiration of gastric juice during operations under general anesthesia (Mendelssohn's syndrome).
Contraindications
- pregnancy;
- lactation;
- children under 12 years old;
- hypersensitivity to ranitidine or other components of the drug.
special instructions
Treatment with ranitidine can mask symptoms associated with gastric carcinoma, therefore, the presence of ulcer cancer must be ruled out before starting treatment.
Ranitidine, like all H2-histamine blockers, is undesirable to abruptly cancel ("rebound" syndrome).
With prolonged treatment of debilitated patients under stress conditions, bacterial lesions of the stomach are possible with the subsequent spread of infection.
There is evidence that ranitidine can cause acute attacks of porphyria.
H2-histamine receptor blockers should be taken 2 hours after taking itraconazole or ketoconazole to avoid a significant decrease in their absorption.
May cause a false positive reaction to a urine protein test.
H2-histamine receptor blockers can counteract the effect of pentagastrin and histamine on the acid-forming function of the stomach, therefore, it is not recommended to use H2-histamine receptor blockers within 24 hours prior to the test.
H2-histamine receptor blockers can suppress the skin reaction to histamine, thus leading to false-positive results (it is recommended to stop using histamine H2-receptor blockers before performing diagnostic skin tests to detect an immediate allergic skin reaction).
During treatment, you should avoid eating foods, drinks and other medicines that can irritate the stomach lining.
Influence on the ability to drive vehicles and use mechanisms
During the period of treatment, it is necessary to refrain from engaging in potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions.
Side effect
- nausea, vomiting;
- dry mouth;
- constipation;
- diarrhea;
- abdominal pain;
- acute pancreatitis;
- leukopenia, thrombocytopenia, agranulocytosis, pancytopenia;
- decline blood pressure;
- bradycardia;
- arrhythmia;
- atrioventricular block;
- increased fatigue;
- drowsiness;
- headache;
- dizziness;
- confusion of consciousness;
- noise in ears;
- irritability;
- hallucinations (mainly in elderly patients and critically ill patients);
- blurred visual perception;
- arthralgia;
- myalgia;
- hyperprolactinemia;
- gynecomastia;
- amenorrhea;
- decreased libido;
- impotence;
- hives;
- skin rash;
- angioedema;
- anaphylactic shock;
- bronchospasm;
- alopecia.
Drug interactions
Smoking reduces the effectiveness of ranitidine.
Increases the concentration of metoprolol in blood serum (by 80% and 50%, respectively), while the half-life of metoprolol increases from 4.4 to 6.5 hours.
Due to an increase in the pH of the contents of the stomach with simultaneous administration, the absorption of itraconazole and ketoconazole may decrease.
Inhibits the metabolism in the liver of phenazone, aminophenazone, diazepam, hexobarbital, propracolol, diazepam, lidocaine, phenytoin, theophylline, aminophylline, indirect anticoagulants, glipizide, buformin, metronidazole, calcium antagonists.
Medicines that suppress the bone marrow increase the risk of neutropenia.
With simultaneous use with antacids, sucralfate in high doses, it is possible to slow down the absorption of ranitidine, therefore, the interval between taking these drugs should be at least 2 hours.
Analogs of the drug Ranitidine
Structural analogues for the active substance:
- Acidex;
- Atsilok;
- Hertocalm;
- Histak;
- Zantak;
- Zantin;
- Zoran;
- Raniberl 150;
- Ranigast;
- Ranisan;
- Ranital;
- Ranitidine Sediko;
- Ranitidine Sopharma;
- Ranitidine Akos;
- Ranitidine Acri;
- Ranitidine-Lect;
- Ranitidine-Ferein;
- Ranitidine hydrochloride;
- Ranitine;
- Rantak;
- Ranks;
- Ulkodin;
- Ulkosan;
- Ulran.
Application in children
Contraindicated in children under 12 years of age.
Application during pregnancy and lactation
Ranitidine is contraindicated during pregnancy and lactation.
Pain in the epigastric region caused by inflammation of the mucous membrane often accompanies peptic ulcers, but it can also be a simple situational heartburn due to increased acidity of the stomach. In such a situation, pharmacists and doctors recommend taking the drug of the antacid category. In particular, little-known "Ranitidine" also gets here. What are these pills from, and how to take them correctly?
What does Ranitidine help with?
By its nature, the drug is an antihistamine, but its real scope of action is somewhat different from simple suppression of allergy manifestations. The active substance, ranitidine hydrochloride, inhibits the H2 receptors in the gastric mucosa, and also reduces the production of hydrochloric acid and affects acidity, reducing its level. Thus, the pharmacology of "Ranitidine" is an antiulcer agent that allows you to relieve the acute symptoms of any lesions of the gastric mucosa: both with gastroduodenitis and ulcers.
- The dosage of the active substance per 1 tablet is 150 and 300 mg.
- After taking 150 mg (single dose), the effect lasts for 12 hours, the maximum concentration is observed 3 hours after ingestion, while food consumption does not affect these indicators.
Ranitidine (as a substance) actively passes into breast milk, and can also affect prolactin levels (temporarily) if it is given by intravenous injection. The duration of elimination depends on the renal function.
General focus this drug - any ulcerative lesions of the mucous membrane, both in chronic and acute form... "Ranitidine" is indicated for:

- prevention of peptic ulcer disease after surgery and stress;
- treatment of Zollinger-Ellson syndrome, esophagitis, ulcerative lesions of the gastrointestinal tract.
Despite the fact that active ingredient quite safe, there are a number of restrictions on taking the drug. "Ranitidine" is not recommended for pregnant and lactating women, as well as children under 12 years of age, people with impaired renal function and liver cirrhosis. In addition, doctors note several more nuances associated with therapy and a single use of the drug.
- Since "Ranitidine" removes acute manifestations ulcerative attacks, it should be applied only after the diagnosis has been made: otherwise there is a risk of not noticing the development of gastric carcinoma or other diseases that have symptoms similar to peptic ulcer disease.
- Long-term therapy should not be carried out in persons who have undergone severe stress and need recovery, since the mucous membrane of the upper gastrointestinal tract may be damaged, which will entail the opposite effect.
- Ranitidine comes into conflict with ketoconazole and itraconazole, as a result of which they are spread in doses for 2-3 hours. In addition, it is undesirable to combine this element with pentogastrin and histamine. Also, therapy based on it requires adherence to a diet that is gentle for the stomach and intestines: the exclusion of spicy, salty, smoked, etc. It is advisable to give up nicotine, since it reduces the effectiveness of treatment.
The effect of "Ranitidine" on the central nervous system is extremely rare - the concentration and reaction rate decrease.

Taking the drug does not depend on the fullness of the stomach and intestines, so the pill can be taken both with meals and after. It is undesirable to chew it, but be sure to drink plenty of liquid room temperature... The dosage is calculated depending on the goals of the treatment.
- For preventive purposes, "Ranitidine" is drunk 1 tablet of 150 mg no more than 2 times a day, the course varies from 14 to 56 days.
- Treatment of peptic ulcer disease is carried out for 10-12 weeks, during which in the morning and in the evening they take 150 mg of the drug, or drink 300 mg at night.
- During the period of preparation for anesthesia with Mendelssohn's syndrome, they drink no more than 150 mg 1.5-2 hours before it. You can additionally take the same amount one day before the operation.
- In case of impaired renal function, it is undesirable to exceed the daily dosage of 150 mg.
- Maximum adult dose - 6 g, used for intravenous administration for the purpose of removing acute symptom... With long-term treatment, it is undesirable to drink more than 300 mg per day.
Adverse reactions to "Ranitidine" were recorded from the nervous, digestive, cardiovascular systems. Allergies on the skin, problems of the musculoskeletal system and breathing are also possible. In case of an overdose, convulsions are observed.
Due to the fact that "Ranitidine" is dispensed only by prescription of a specialist, if the instructions are followed, the likelihood of a negative reaction to it decreases, but doctors still recommend monitoring the state of the body in the first days, and if any side effects appear, seek adjustment of therapy.
P N014316 / 01Tradename: Ranitidine
International non-proprietary name:
RanitidineDosage form:
film-coated tabletsStructure
Composition for one tablet:
active substance: ranitidine 150.00 mg (in the form of ranitidine hydrochloride - 167.40 mg);
auxiliary substances: microcrystalline cellulose. croscarmellose sodium, colloidal silicon dioxide, magnesium stearate; shell: hypromellose, grnacetin, titanium dioxide E 171, talc.
Description
round biconvex tablets, film-coated from white to yellowish-white, with a characteristic odor. On a cross section, the kernel is from almost white to white with a yellowish tinge.
Pharmacotherapeutic group:
an agent that lowers the secretion of gastric glands - H 2 - histamine receptor blocker.ATX code: [А02ВА02]
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics. Ranitidine is a blocker of H 2 -histamine receptors of parietal cells of the gastric mucosa. Reduces basal and stimulated secretion of hydrochloric acid caused by irritation of baroreceptors, food load, the action of hormones and biogenic stimulants (gastrin, histamine, acetylcholine, pentagastrin, caffeine). Ranitidine reduces the volume of gastric juice and the content of hydrochloric acid in it, increases the pH of the stomach contents, which leads to a decrease in the activity of pepsin. The duration of action after a single dose is up to 12 hours.
Pharmacokinetics. It is rapidly absorbed from the gastrointestinal tract; food intake does not affect the degree of absorption. When administered orally, the bioavailability of ranitidine is 50%. The connection with plasma proteins does not exceed 15%. Partially metabolized in the liver. The maximum plasma concentration is reached 2 hours after taking the coated tablets and ranges from 36 to 94 ng / ml. The half-life is 2-3 hours. 35% of the dose taken is excreted in the urine unchanged, a small amount in the feces.
Penetrates the placenta. It is excreted in breast milk (the concentration in breast milk in a woman during lactation is higher than in plasma.)
Indications for use
Treatment and prevention of exacerbations of gastric ulcer and duodenal ulcer; ulcers of the stomach and 12 duodenal ulcers associated with the use of non-steroidal anti-inflammatory drugs (NSAIDs); reflux esophagitis, erosive esophagitis; Zollinger-Ellison syndrome; treatment and prevention of postoperative, "stress" ulcers of the upper gastrointestinal tract (GIT); prevention of recurrence of bleeding from the upper gastrointestinal tract; prevention of aspiration of gastric juice during operations under anesthesia (Mendelssohn's syndrome).Contraindications
Hypersensitivity to ranitidine or other components of the drug.Pregnancy, lactation period. Childhood up to 14 years old.
Carefully
Renal and / or hepatic failure, cirrhosis of the liver with a history of portosystemic encephalopathy; acute porphyria (including a history).
Method of administration and dosage
Inside, regardless of food intake, without chewing, drinking a small amount of liquidPeptic ulcer and 12 duodenal ulcer. For the treatment of exacerbations, 150 mg are prescribed 2 times a day (morning and evening) or 300 mg at night. If necessary, 300 mg 2 times a day. The duration of the course of treatment is 4–8 weeks. For the prevention of exacerbations, 150 mg is prescribed at night.
Ulcers associated with taking NSAIDs. Assign 150 mg 2 times a day or 300 mg at night for 8-12 weeks. Prevention of ulceration when taking NSAIDs - 150 mg 2 times a day.
Postoperative and stress ulcers. Assign 150 mg 2 times a day for 4-8 weeks.
Gastroesophageal reflux disease. Assign 150 mg 2 times a day or 300 mg at night. If necessary, the dose can be increased to 150 mg 4 times a day. The course of treatment is 8-12 weeks.
Zollinger-Ellison Syndrome. The initial dose is 150 mg 3 times a day, if necessary, the dose can be increased to 6 g / day.
Prevention of recurrent bleeding. 150 mg 2 times a day.
Prevention of the development of Mendelssohn's syndrome. Ranitidine is prescribed at a dose of 150 mg 2 hours before anesthesia, and preferably 150 mg the night before.
For patients with renal failure with creatinine clearance less than 50 ml / min, the recommended dose is 150 mg per day.
Side effect
From the digestive system: nausea, dry mouth, constipation, vomiting, diarrhea, abdominal pain; rarely - hepatitis (hepatocellular, cholestatic, mixed), acute pancreatitis.From the side of the hematopoietic organs: leukopenia, thrombocytopenia, agranulocytosis, pancytopenia, bone marrow hypo- and aplasia, immune hemolytic anemia.
On the part of the cardiovascular system: lowering blood pressure, bradycardia, vasculitis, arrhythmia, atrio-ventricular block.
From the nervous system: increased fatigue, headache, dizziness, drowsiness, insomnia, emotional lability, anxiety, anxiety, depression, hyperthermia; rarely - confusion, tinnitus, irritability, hallucinations (mainly in elderly patients and seriously ill patients), involuntary movements.
From the senses: blurred vision, paresis of accommodation.
From the side of the musculoskeletal system: arthralgia, myalgia.
From the endocrine system: hyperprolactinemia, gynecomastia, amenorrhea, decreased potency and / or libido.
Allergic reactions: urticaria, skin rash, pruritus, angioedema, anaphylactic shock, bronchospasm, exudative erythema multiforme (including Stevens-Johnson syndrome).
Others: alopecia, hypercreatininemia, increased activity of glutamate transpeptidase, acute porphyria.
OVERDOSE
Symptoms: convulsions, bradycardia, ventricular arrhythmias.
Treatment: symptomatic. With the development of seizures - intravenous diazepam, with bradycardia or ventricular arrhythmias - atropine, lidocaine. Hemodialysis is effective.
Interaction with other medicinal products
Due to an increase in the pH of the contents of the stomach, while taking it, the absorption of itraconazole and ketoconazole may decrease.Inhibits metabolism in the liver of phenazone, aminophenazone, diazepam, hexobarbital, propranolol, metoprolol, nifedipine, warfarin, diazepam, lidocaine, lignocaine, phenytoin, theophylline, aminophylline, indirect anticoagulants, gminipizide, bufizide.
With simultaneous use with procainamide, an increase in its concentration in blood plasma is possible due to a decrease in its excretion by the kidneys.
With simultaneous use with antacids, sucralfate in high doses, the absorption of ranitidine may be impaired, so the interval between taking these drugs should be at least 2 hours.
Medicines that suppress the bone marrow increase the risk of neutropenia. Smoking reduces the effectiveness of ranitidine.
SPECIAL INSTRUCTIONS
Treatment with ranitidine can mask the symptoms associated with gastric carcinoma, therefore, before starting treatment, it is necessary to exclude the presence of a malignant neoplasm. Ranitidine, like all blockers of H 2 -histamine receptors, is undesirable to abruptly cancel (in order to avoid the "rebound" syndrome).
With prolonged treatment of debilitated patients under stress conditions, bacterial lesions of the stomach are possible, followed by the spread of infection.
There is evidence that ranitidine can cause acute attacks of porphyria.
During the period of treatment, it is necessary to refrain from engaging in potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions.
May increase the activity of glutamate transpeptidase.
May cause false positives when testing for protein in urine. Blockers of H 2 -histamine receptors can counteract the effect of pentagastrin and histamine on the acid-forming function of the stomach, therefore, during the 24 hours preceding the test, their use is not recommended.
May suppress skin reaction to histamine, thus leading to false positive results (it is recommended to stop taking ranitidine before performing diagnostic skin tests to detect an immediate allergic skin reaction).
During treatment, avoid eating food, beverages and other medicines that can irritate the stomach lining
Release form
Film-coated tablets, 150 mg. 6 or 10 tablets in a blister of aluminum foil and aluminum foil, laminated with nolivinyl chloride and polyamide film.
5 blisters each but 6 tablets or 3 blisters of 10 tablets each with instructions for medical use medicinal product in a cardboard box.
STORAGE CONDITIONS
At a temperature not exceeding 25 ° С in consumer packaging.
Keep out of the reach of children.
SHELF LIFE
3 years.
Do not use after the expiration date printed on the package.
HOLIDAY FROM PHARMACIES
Dispensed by prescription
Manufacturer
Hemofarm A.D. Vrsac, branch production site Sabac, Serbia
15000, Sabac, st. Hajduk Velkova 66
Marketing authorization holder / packer / packer / issuing quality control
Hemofarm A.D .. Serbia 26300, Vrsac. Beogradsky Way 66
(lat. ranitidine) - antiulcer drug, blocker of histamine H2 receptors. Historically, the second (after cimetidine) antisecretory drug that suppresses acid production in the stomach. It is now considered to be relatively old, with more side effects than current drugs and less efficacy than famotidine and proton pump inhibitors.
In the fall of 2019, a number of drugs containing ranitidine were banned or approved for use in clinical practice was temporarily suspended by regulators in many countries due to the presence or due to suspicion of the presence (the latter was motivated by unsatisfactory testing of the drug for this ingredient) of a potential carcinogen - N-nitrosodimethylamine.
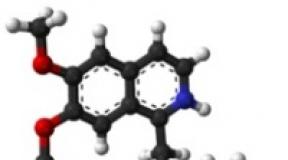
Ranitidine is a chemical compound
N-thio] ethyl] -N "- methyl-2-nitro-1,1-ethenediamine hydrochloride. Empirical formula: C 13 H 22 N 4 O 3 S.
Ranitidine - medicinal product
Ranitidine - international non-proprietary name (INN) of the medicinal product. According to the pharmacological index, it belongs to the group "Blockers of H2-histamine receptors II generation ". According to ATX - to the group "H2-histamine receptor blockers" and has the code A02BA02.Pharmacodynamics of ranitidine
Ranitidine is a blocker of histamine H2 receptors of parietal cells of the gastric mucosa. Reduces basal and stimulated secretion of hydrochloric acid caused by irritation of baroreceptors, food stress, the action of hormones and biogenic stimulants (gastrin, histamine, pentagastrin). Ranitidine reduces the volume of gastric juice and the content of hydrochloric acid in it, reduces the acidity of the stomach, which leads to a decrease in the activity of pepsin. The duration of action of ranitidine after a single dose is up to 12 hours.Pharmacokinetics of ranitidine
 When administered orally, the bioavailability of ranitidine is 50%. Plasma protein binding does not exceed 15%. Partially metabolized in the liver. The maximum plasma concentrations of ranitidine are reached 2 hours after taking the coated tablets, 1 hour after taking effervescent tablets and range from 36 to 94 ng / ml. The half-life is 2-3 hours. About 30% of the taken dose of ranitidine is excreted in the urine unchanged, a small amount in the feces. Penetrates the placenta. Excreted in breast milk.
When administered orally, the bioavailability of ranitidine is 50%. Plasma protein binding does not exceed 15%. Partially metabolized in the liver. The maximum plasma concentrations of ranitidine are reached 2 hours after taking the coated tablets, 1 hour after taking effervescent tablets and range from 36 to 94 ng / ml. The half-life is 2-3 hours. About 30% of the taken dose of ranitidine is excreted in the urine unchanged, a small amount in the feces. Penetrates the placenta. Excreted in breast milk. Professional medical work affecting the treatment of gastrointestinal diseases with ranitidine
- Gorbakov V.V., Makarov Yu.S., Golochalova T.V. Comparative characteristics of antisecretory drugs of various groups according to daily pH monitoring // Attending physician. 2001. - No. 5-6.
- Yakovenko E.P. Zantac in the treatment of acid-related diseases. Russian State Medical University, Federal Gastroenterological Center, Moscow.
- Makhakova G.Ch., Dicheva D.T., Odintsova T.A. and other Comparative characteristics of acid-suppressing drugs by conducting pharmacological tests with intragastric daily pH-metry // Attending physician. - 1999. - No. 6. - P. 24–26.
Indications for the use of ranitidine

Dosing and administration of ranitidine
- Peptic ulcer of the stomach and duodenum. For the treatment of exacerbations, 150 mg of ranitidine is prescribed 2 times a day (morning and evening) or 300 mg at night. If necessary, 300 mg 2 times a day. The duration of the course of treatment is 4-8 weeks. For the prevention of exacerbations, 150 mg is prescribed at night.
- Ulcers associated with taking NSAIDs. Assign 150 mg of ranitidine 2 times a day or 300 mg at night for 8-12 weeks. Prevention of ulceration when taking NSAIDs - 150 mg 2 times a day.
- Postoperative ulcers. Assign 150 mg of ranitidine 2 times a day for 4-8 weeks.
- Gastroesophageal reflux disease. Assign 150 mg 2 times a day or 300 mg at night. If necessary, the dose can be increased to 150 mg 4 times a day. The course of treatment is 8-12 weeks.
- Zollinger-Ellison Syndrome. The initial dose is 150 mg of ranitidine 3 times a day, if necessary, the dose can be increased.
- Prevention of recurrent bleeding. 150 mg 2 times a day.
- Prevention of the development of Mendelssohn's syndrome. Ranitidine is prescribed at a dose of 150 mg 2 hours before anesthesia, and preferably 150 mg the night before.
 Ranitidine is taken regardless of food intake, without chewing, with a small amount of liquid.
Ranitidine is taken regardless of food intake, without chewing, with a small amount of liquid. Effervescent tablets are dissolved in a glass of water and drunk after complete dissolution.
For patients with renal failure with creatinine clearance less than 50 ml / min, the recommended dose is 150 mg of ranitidine per day.
Precautions when using ranitidine
- It is undesirable to abruptly stop taking ranitidine because of the danger of recurrence of peptic ulcer disease.
- Ranitidine is used with caution in patients with impaired renal and hepatic function.
- Before starting treatment with ranitidine, it is necessary to exclude the possibility of a malignant disease of the esophagus, stomach or duodenum.
Contraindications to the use of ranitidine
Hypersensitivity to ranitidine or other components of the drug. Pregnancy, lactation. Children under 14 years of age.Side effects of ranitidine
- From the nervous system and sensory organs: headache, fatigue, dizziness, drowsiness, insomnia, vertigo, anxiety, depression; rarely - confusion, hallucinations (especially in elderly and debilitated patients), reversible blurred vision, impaired eye accommodation.
- From the side of the cardiovascular system and blood (hematopoiesis, hemostasis): arrhythmia, tachycardia, bradycardia, AV blockade, decreased blood pressure; reversible leukopenia, thrombocytopenia, granulocytopenia; rarely - agranulocytosis, pancytopenia, sometimes with bone marrow hypoplasia, aplastic anemia; sometimes - immune hemolytic anemia.
- From the digestive tract: nausea, vomiting, constipation, diarrhea, abdominal discomfort, pain; rarely - pancreatitis. Sometimes - hepatocellular, cholestatic or mixed hepatitis with / without jaundice (in such cases, the reception of ranitidine must be stopped immediately). These effects are usually reversible, but in rare cases, death is possible. There have also been rare cases of liver failure. In healthy volunteers, the concentration of AST was increased at least 2 times in relation to the level before treatment in 6 of 12 people who received 100 mg 4 times / in / for 7 days, and in 4 of 24 people who received 50 mg 4 times i.v. for 5 days.
- From the side of the musculoskeletal system: rarely - arthralgia, myalgia.
- Allergic reactions: skin rash, bronchospasm, fever, eosinophilia; rarely - erythema multiforme, anaphylactic shock, angioedema.