China Instructions for use. Not available. International non-proprietary title
Lancide Whale: Instructions for use and reviews
Latin name: Lancid Kit.
ATX code: A02BD
Active substance: Clarithromycin (Clarithromycin) + Amoxicillin (Amoxicillin) + Lansoprazole (Lansoprazole)
Manufacturer: Micro Labs LIMITED (India)
Actualization of the description and photo: 30.11.2018
Whale Lancide is a means for the treatment of peptic ulcers.
Release form and composition
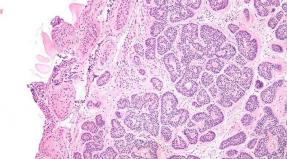
Whale Lancide is a set of tablets and capsules [in a blister, a distinted perforated line into two parts (morning and evening), 2 tablets of clarithromycin, 4 amoxicillin capsules and 2 lansoprazole capsules; In a cardboard pack of 7 blisters and instructions for the use of Lancide Kit]:
- tablets coated with film shell containing clarithromycin: oval shape, with a risk on one of the sides, yellow, on a bend - white color;
- capsules containing amoxicillin: Size number 0, solid gelatin, with a chip of yellow and dark red, with black inscription "500" on the housing and "amoxi" on the lid; Content - crystalline powder of almost white or white;
- capsules containing Lansoprazole: Size number 1, solid gelatin, with a housing and a pink cover, with a black inscription "Micro / Micro"; Content - Granules (Pellets) of almost white or white.
Composition 1 Clarithromycin tablets:
- active substance: clarithromycin - 500 mg;
- auxiliary components: sodium croscarmelloz, silicon colloidal dioxide, corn, microcrystalline starch, stearic acid, sorbitan oleate, sorbic acid, magnesium stearate, povidone, talc;
- film shell: propylene glycol, hypimosellos, titanium dioxide (E171), chinoline yellow dye (E104), vanilla flavor.
Composition 1 amoxicillin capsules:
- active substance: amoxicillin (in the form of trihydrate) - 500 mg;
- auxiliary components: sodium laurylsulfate, talc, magnesium stearate;
- capsule shell: housing - gelatin, methyl aprichedroxybenzoate, propyl aprichedroxybenzoate, titanium dioxide (E171), yellow oxide (E172); Lid - gelatin, water, methylpaultydroxybenzoate, propyl aprichedroxybenzoate, titanium dioxide (E171), dye solar sunset yellow (E110), diamond blue dye (E133);
- black ink: isopropanol, shellac, butanol, ethanol, propylene glycol, ammonia aqueous, iron dye oxide black (E172).
Composition 1 Lancel Capsules:
- active substance: Lansoprazole - 30 mg;
- auxiliary components: diethyl phthalate, sodium hydroxide, calcium carmellosis, sucrose, microspheres made of sucrose, hypimon, mannitol, polysorbate 80, magnesium hydroxycarbonate, sodium hydrophosphate, povelne, talc, titanium dioxide (E171), methacrylic acid copolymer (type A);
- capsule shell: housing - gelatin, propyl aprichedroxybenzoate, methyl aprichedroxybenzoate, sodium laurylsulfate, water, titanium dioxide (E171), Punchy dye (punching 4R) (E124); Lion - gelatin, propyl aprichedroxybenzoate, sodium Laurilsulfate, water, methylpaultydroxybenzoate, titanium dioxide (E171), Punchy dye (punching 4R) (E124);
- black ink: butanol, isopropanol, shellac, propylene glycol, ethanol, ammonia aqueous, iron dye oxide black (E172).
Pharmacological properties
Pharmacodynamics
Whale Lancide is a combined composition preparation used for Helicobacter Pylori eradication. The mechanism of action is due to the properties of active ingredients.
Clarithromycin
Clarithromycin is a semi-synthetic derivative of erythromycin A, a bacteriostatic macrolide antibiotic. The antibacterial effect is a consequence of the binding of 50s subunit membrane ribosomes of the microbial cell and suppressing the synthesis of protein of microorganisms.
It is active in respect of a large number of aerobic and anaerobic gram-negative and gram-positive microorganisms, including Helicobacter Pylori bacterium.
Amoxicillin
Amoxicillin - semi-synthetic penicillin with bactericidal effect wide spectrum actions. The antibiotic has the ability to inhibit transpeptide, disrupt the synthesis of peptideoglycan (cell wall support protein) during the division and growth period, cause lysis bacteria. Exhibits high activity against Helicobacter Pylori.
The combination of clarithromycin + amoxicillin has a potentiated antimicrobial effect on H. pylori.
Lansoprazole
Lansoprazole is a specific proton pump inhibitor (H + / K + -ATF-AZA). In the stomach parietal cells, biotransformed to active sulfonamide derivatives, which inactivate H + / K + -TF-AZU. Reduces basal and stimulated secretion (regardless of the nature of the irritant), due to which blocks the final stage of secretion of hydrochloric acid. It has high lipophilicity, so it easily penetrates into parietal stomach cells and concentrated in them. Increases the oxygenation of the stomach mucosa and increases the secretion of the hydrocarbonate, as a result of which the cytoprotective effect has.
The rate and degree of suppression of basal and stimulated secretion of hydrochloric acid are dose-dependent: pH starts to increase after 1-2 hours and 2-3 hours after taking Lansoprazole in a dose of 15 and 30 mg, respectively. When receiving a dose of 30 mg, the braking of hydrochloric acid products is 80-97%.
It does not affect the motorcy of the gastrointestinal tract.
The inhibitory effect increases in the first 4 days of reception of the drug. After the abolition of Lansoprazole for 39 hours, the acidity remains below 50% of the basal level. A ricketer increase in secretion is not marked. Secretor activity is normalized after 3-4 hours after canceling the drug.
In the syndrome of Zolinger - Ellison acts longer.
Suppressing the growth of Helicobacter Pylori, contributes to the formation of a stomach-specific IGA in the gastric mucous membrane to bacteria. Increases the antihelicobacter activity of others medicinal preparations. Inhibits pepsin production and increases plasma concentration of pepsinogen. The suppression of secretion is accompanied by an increase in the number of nitrosobacteria and the concentration of nitrates in the gastric secrecy.
The drug is effective for ulcer of stomach and duodenal gutresistant to Blockers H 2 -Histamine receptors. Provides more rapid healing of ulcer lesions in the duodenum (85% of duodenal ulcers heal for 4 weeks of regular lanceprazole reception in a daily dose of 30 mg).
Pharmacokinetics
Clarithromycin
The substance is quick and well absorbed from the gastrointestinal tract. Absolute bioavailability is approximately 50%. Simultaneous food intake slightly slows down the suction of clarithromycin, but does not have a significant effect on bioavailability. With repeated reception, there is no cumulating in the body, the character of metabolism does not change.
About 80% dose is associated with plasma proteins. After a single reception, there are 2 peaks of the maximum concentration (C MAX). The second is explained by the accumulation of clarithromycin in bile bubble, subsequent entry into the intestines and suction. With a single reception of a dose of 500 mg C MAX is achieved within 2-3 hours.
Metabolized with the participation of the CYP3A isoenzyme of the cytochrome R 450 system. It is the inhibitor of CYP3A4, CYP3A5 and CYP3A7 isoenzymes.
Approximately 20% of the adopted dose is rapidly hydroxylates in the liver to form 14 (R) -hydroxiclarithromycin - the main metabolite, which also has a pronounced antimicrobial activity.
With an equilibrium state, the concentration of metabolite does not increase in proportion to doses of clarithromycin. The half-life (T ½) periods of clarithromycin and 14 (R) -hydroxiclarithromycin increase with an increase in the adopted dose.
When using clarithromycin in higher doses, there is a decrease in the formation of 14 (R)-hydroxyclarithromycin and N-demethylated metabolites, which indicates a nonlinear nature of pharmacokinetics of the drug.
With regular use of the drug in a daily dose of 500 mg, equilibrium concentrations (C SS) in the blood plasma of unchanged clarithromycin and its main metabolite are respectively 2.7-2.9 μg / ml and 0.83-0.88 μg / ml, T ½ - respectively, 4.8-5 h and 6.9-8.7 h.
In therapeutic doses, clarithromycin accumulates in the lungs, skin and soft tissues. In soft concentration tissues 10 times higher than in blood plasma.
The drug is excreted by the kidneys and through the intestines: unchanged - 20-30%, the rest is in the form of metabolites.
Amoxicillin
Quickly and well absorbed from the gastrointestinal tract. The degree and speed of suction do not depend on meals. The acidic stomach environment does not have a destructive effect. Absolute bioavailability is dose-dependent in nature and is 75-90%.
After a single reception of a dose of 500 mg C Max is 6-11 mg / l and is observed after 1-2 hours. 17% is associated with plasma proteins.
Amoxicillin penetrates through histohematic barriers, with the exception of unchanged blood-behaneencephalical. Well distributed in tissues: in high concentrations is formed in the blood plasma, pleural and peritoneal fluids, the bronchial secrete (in the purulent bronchial secretion, the distribution is weak), sputum, tissue, the urine, intestinal mucous membrane, gallbladder (with normal liver function), fatty fabrics, bones, medium ear liquid (in its inflammation), the contents of the skin blisters, prostate gland, female genital organs. Penetrates the placenta, in small quantities - in breast milk. In the event of an increase in the dose of amoxicillin, a proportional increase in concentration in tissues and organs is noted. The content in bile in 2-4 is higher than in plasma. Amoxicillin concentration in amniotic fluid and umbilical vessels is 25-30% of the concentration in the plasma of the blood of a pregnant woman. In small quantities, amoxicillin is highlighted with breast milk.
The drug penetrates poorly through the hematostephalic barrier. In patients with meningitis (inflammation brain shells) Concentration of substance in spinal fluid It is approximately 20% of the plasma level.
Approximately 7-25% dose of amoxicillin are subjected to biotransformation, as a result of which inactive penicillic acid is formed.
Displays: liver - 10-20%, kidneys - 50-70% mainly unchanged by the channel secretion (80%) and glomerular filtration (20%). T ½ is 1-1.5 hours. In patients with a disruption of the renal function [Creatine clearance (CC) less than 15 ml / min] T ½ increases to 5-20 hours. The drug is derived during hemodialysis.
Lansoprazole
Quickly and well absorbed from the gastrointestinal tract. Bioavailability - 80-90%. With simultaneous meals, the absorption and bioavailability are twice as well, but the inhibitory effect of Lansoprazole on the gastric secretion does not change regardless of food intake. Suction can be slowed down in cirrhosis of the liver.
C MAX and AUC (area under the concentration - time curve) approximately proportional. When taking the drug 30 minutes after meals, both pharmacokinetic indicators decrease by 50%. Food does not have a significant effect when taking the drug before meals.
With plasma proteins, a 97% dose binds, the relationship may decrease by 1-1.5% with a violation of the kidney function.
After receiving lansoprazole at a dose of 30 mg C Max, it is 0.75-1.15 mg / l and is achieved within 1.5-2 hours. The drug penetrates well into the tissue, including the chopping cells of the stomach. The distribution volume is 0.5 l / kg.
Lansoprazole is exposed to active metabolism at the first pass through the liver. Biotransformed with the participation of the CYP2C19 isoenzyme and, possibly, CYP3A4, as a result of which metabolites are formed, from which two-hydroxylate sulfinyl and sulfon derivatives are found in significant amounts in plasma, which are inactive. In the acidic medium of the steaming of parietal cells, the drug is biofraught into two active metabolite, but they are not detected in systemic blood flow.
After the first reception of 30 mg of Lansoprazole pH of the gastric juice rises after 1-2 hours. When applying a drug of 30 mg several times a day, this figure is increased in the first hour after the reception. The effect is maintained for more than 24 hours. normal level The secretion of hydrochloric acid is restored in 2-4 days after the cessation of several doses of Lansoprazole.
T ½ is 1-2 hours, in old age increases to 1.9-2.9 hours, in patients with impaired liver function - up to 3.2-7.2 hours.
The drug is derived from the body in the form of Lansoprazola sulfone and hydroxylansoprazole: with bile - ⅔, kidneys - 14-23%. In renal failure, the speed and the magnitude of the excretion do not significantly change.
Indications for use
Lancide whale is intended for the treatment of ulcer of the stomach and duodenum, including for the eradication of Helicobacter Pylori infection.
Contraindications
Absolute:
- lack of sugar / isomaltase, fructose intolerance, glucose-galactose malabsorption;
- severe liver failure that occurs simultaneously with renal failure;
- diseases of the gastrointestinal tract in history, especially colitis associated with the use of antibiotics;
- porphyry;
- pollnosis;
- bronchial asthma;
- atopic dermatitis;
- infectious mononucleosis;
- lympholoicosis;
- cholestatic jaundice / hepatitis in history, developed with clarithromycin;
- hypokalemia;
- the elongation of the Qt interval, ventricular arrhythmia or ventricular tachycardia by the type "Pirouette" in history;
- pregnancy and breastfeeding period;
- age up to 18 years;
- simultaneous use of the following drugs: Terfenadine, Pimozide, Cispanid, Asthemisol, Argotian alkaloids (for example, ergotamine, dihydroeergotamine), Midazolam in oral administration dosage forms, GMG-Coa-reductase inhibitors (statins), which are largely metabolized with CYP3A4 (Lovastatin, Simvastatin), and colchine in patients with impaired kidney and liver function;
- hypersensitivity to any component of the Lancide Whale, Penicillins, macrolides, carbapenes or cephalosporins.
Relative (whale lancide should be used with caution):
- miastic gravis;
- bleeding in history;
- hepatic / renal insufficiency of medium and severe;
- pronounced bradycardia (less than 50 hp / min), severe heart failure, ischemic heart disease;
- hypomagnation;
- allergic reactions, in t. In anamnesis;
- elderly age;
- simultaneous reception of clopidogrel, benzodiazepines (midazolam intravenously, triazole, alprazolam), calcium channel blockers, metabolized by the CYP3A4 isoenzyme (for example, amlodipine, verapamil, diltiazem);
- the need to use drugs that are metabolized by the CYP3A isoenzyme ( indirect anticoagulantov, cyclosporine, tacrolimus, methylprednisolone, dyspeciramide, carbamazepine, silildenafil, chinidine, omeprazole, rifabutin, cylostazol, vinblastine);
- the combined purpose of drugs induced by the CYP3A4 isoenzyme (for example, carbamazepine, rifampicin, phenobarbital, phenytoin, hormour of the junged);
- concomitant therapy with antiarrhythmic drugs IA class (quinidine, procanamide) and class III (dfethylide, amiodarone, sotalol).
Lancide Kit, Application Instructions: Method and Dosage
Lancide whale should be taken inside, before meals, 1 tablet of clarithromycin (500 mg), 2 amoxicillin capsules (1000 mg) and 1 capsule of lansoprazole (30 mg) twice a day, in the morning and in the evening. Tablets and capsules should be swallowed entirely, not smoking and not chewing, drinking enough water.
The course of treatment is 7 days, if necessary, duration increases to 14 days.
Side effects
Information on unwanted drug reactions Lancide Whale is missing. Below are described side effectspeculiar to each of the active substances of the drug. In the frequency of occurrence, they are classified as follows: Very often - ≥ 1/10, often - from ≥ 1/100 to< 1/10, нечасто – от ≥ 1/1000 до < 1/100, редко – от ≥ 1/10 000 до < 1/1000, очень редко – < 1/10 000, частота неизвестна – на основании имеющихся данных нет возможности оценить интенсивность возникновения побочных реакций.
Overdose symptoms caused by the presence of clarithromycin in the preparation of Lancide Whale: Abdominal pain, diarrhea, nausea, vomiting, headache, confusion of consciousness. Treatment: Stomaching and maintenance of supporting therapy. Hemodialysis and peritoneal dialysis are ineffective.
Overdose symptoms caused by the presence of amoxicillin in the preparation: crystalluria, diarrhea, nausea, vomiting, violation of the water and electrolyte balance (effect of diarrhea and vomiting). Treatment: washing the stomach, the reception of activated carbon, salt laxatives and preparations for maintaining the water and electrolyte balance. Hemodialysis can be used to remove amoxicillin from the body.
Cases of overdose of Lansoprazole are not described.
special instructions
Each Blister Lancide Whale is designed for one day of treatment, packaging (7 blisters) - for one 7-day course.
Prior to the beginning of the preparation of the drug, a patient should be examined to eliminate the malignant process (especially with the ulcer of the stomach), since anti-siza therapy can mask symptoms and, as a result, to delay the timely diagnosis.
Clarithromycin
As a result long use antibiotics is possible formation of colonies with increased amount insensitive microorganisms. In the event of the development of superinfection, appropriate treatment is prescribed.
It is possible to develop crosstural resistance to clarithromycin, other macrolide antibiotics, clindamycin and lincomycin.
With the development of acute well-sensitivity reactions (drugs with eosinophilia and systemic symptoms, toxic epidermal necrolis, anaphylaxis, Stevens - Johnson syndrome, Purple Schuhenna - Genoch) should immediately cancel Lancium whale and start conducting appropriate therapy.
There are cases of liver dysfunction (including heavy) on the background of clarithromycin intake. It is usually reversible, but requires the abolition of antibiotic therapy. In the presence of serious diseases in a patient or simultaneous reception of some drugs, hepatic insufficiency is possible with death. Patients should be prevented about the need to appeal for medical help In the case of their signs of hepatitis, such as abdominal diseases during palpation, urine darkening, anorexia, jaundice.
Patients S. chronic diseases Liver during the reception of the Lancide whale should be regularly monitored by blood plasma enzymes.
Like all antibacterial agents, clarithromycin can cause pseudomambranous colitis of varying severity - from light to threatening life. The antibiotic can affect the normal intestinal microflora, which is why the growth of Clostridium difficile increases. In the event of diarrhea, the patient should always suspect a pseudommbranous colitis caused by Clostridium difficile until another will be installed. In this regard, during antibiotic therapy and within 2 months after its end, it is necessary to establish a thorough medical monitoring of the patient.
Caution Clarithromycin should be used in hypomagnalionia, severe heart failure, severe bradycardia, ischemic Disease Hearts simultaneously use of antiarrhythmic media Ia and III class. In all these cases, it is necessary to control the electrocardiogram for an increase in the Qt interval.
There are cases of exacerbation of symptoms of miastic gravis in patients receiving clarithromycin.
With the joint use of warfarin or other indirect anticoagulants, prothrombin time monitoring is needed and many.
Amoxicillin
Cases of the development of serious hypersensitivity reactions on penicillins are described, sometimes with fatal outcome. The risk of their occurrence is the most high in patients who have such reactions took place in the past. In this regard, before the appointment of Amoxicillin, the doctor must collect a detailed history of the patient for the reactions of increased sensitivity on penicillins, cephalosporins or other allergens. When allergic reactions appear against the background of antibiotic therapy, it is necessary to stop taking the drug and immediately take appropriate measures that may include the introduction of epinephrine, intravenous administration glucocorticosteroids, carrying out oxygen therapy, ensuring passability respiratory tract (If necessary - intubation).
With caution, the antibiotic should be used in the presence of allergic diathesis, bronchial asthmaas well as in the case of anamnestic data on colitis caused by antibacterial therapy.
With prolonged use of the drug there is a possibility of excessive reproduction of insensitive microorganisms.
During the application of Lancide, KIT is recommended to periodically check the functions of blood formation, liver and kidney. In patients with functional liver disorders, the body's function monitoring should be carried out on a regular basis. For functional disorders Kidney dose of amoxicillin should be reduced depending on the degree of violations.
It is necessary to take into account the probability of the development of superinfection (usually caused by the mushrooms of the genus Candida or the bacteria of the genus Pseudomonas SPP.), Which requires the abolition of the antibiotic and carry out appropriate treatment.
In diarrhea, which remains for some time, should always suspect the development of pseudommabranous colitis - the disease that is caused by antibiotics and may be dangerous for the patient's life. Its main signs are: a watery feces with a mixture of mucus and blood, chic-shaped or stupid common abdominal pain, fever, sometimes - tenesms (pulling, cutting, burning pain in the area of \u200b\u200bthe rectum). When described symptoms appear, amoxicillin should be canceled and urgently start conducting specific therapy (for example, the use of vancomycin). The use of medicines that reduce the peristaltics of the gastrointestinal tract is contraindicated.
With a reduced diurea, the drug can cause crystallurium. For this reason, in the period of receiving the Lancide Kit, it is important to use a sufficient amount of liquid and maintain sufficient diuresis. With cholant and cholecystitis, the drug can be prescribed only with a mild disease and, subject to the absence of a concomitant cholestasis.
Amoxicillin in large quantities is derived from the body with urine, as a result of which false-positive results can be obtained when determining the content of glucose in the urine (for example, when conducting a Benedict or Feling sample). If necessary, a glucose oxidase method should be applied to the level of glucose in the urine.
Amoxicillin sometimes causes nonspecific binding of albumin and immunoglobulins with the erythrocyte membrane, which is why there is a false positive reaction when conducting a Cumbac sample.
In patients receiving warfarin or other indirect anticoagulants, it is necessary to monitor the prothrombin time and in the period of use of amoxicillin and after its cancellation.
The antibiotic can reduce the effectiveness of estrogen-containing oral contraceptives. During his reception, it is recommended to use an additional reliable method of contraception.
In the case of prolonged use of amoxicillin, the simultaneous purpose of Lev-Room, Nistatin or other antifungal drugs is required.
Lansoprazole
With prolonged use of proton pump inhibitors, the probability of infection is increasing (including Campylobacter, Salmonella and Clostridium Difficile), as well as fractures in women during menopause.
Before appointing the drug should compare the benefit of the prevention of bleeding from the upper departments of the gastrointestinal tract with possible risk Development of fan-associated pneumonia.
In patients receiving warfarin or other indirect anticoagulants, you need to control the prothrombin time and many.
When conducting therapy, alcoholic beverages should not be used.
The effect of lansoprazole depends on the genetic polymorphism of CYP2C19. In patients who are slow metabolizers (RM-type), the effect of the drug above is significantly more often possible to achieve the eradication of Helicobacter Pylori than in patients who are fast metabolizers (HOMEM-type), even with sustainability of claritromycin.
Impact on the ability to control vehicles and complex mechanisms
Whale Lancide can cause dizziness, drowsiness and weakness. In the period of therapy, precautions should be observed when driving a car and occupying other potentially hazardous species Activities requiring concentration of attention and reaction rate.
Application during pregnancy and lactation
Whale Lancide is contraindicated with pregnant and nursing breasted women.
Childcare
The drug does not apply to pediatrics (for the treatment of children and adolescents under 18).
With violations of the kidney function
The drug is contraindicated in patients with renal failure flowing against the background of severe liver failure.
With caution, whale lancide should be used in renal failure of the average and severe degree.
When violations of the liver function
The drug is contraindicated in patients who have severe liver failure proceeding simultaneously with renal failure.
With caution, whale lancide should be used with medium and severe liver failure.
Application in old age
In the elderly, Lancide Kit needs to be taken with caution.
Medicinal interaction
Clarithromycin
Unlikely, clinically significant interaction of clarithromycin with benzodiazepines, the removal of which does not depend on the CYP3A4 isoenzymes (for example, with the tempozepam, nitrazepam, the Lorazepam).
Requests the simultaneous use of the following drugs: Indirect anticoagulants (including warfarin), Valproic acid, Dizeciramide, cyclosporin, carbamazepine, omeprazole, vinblastine, methylprednisolone, cylostazol, phenyotine, rifabutin, simvastatin, chinidine, teofiline, tacrolimus, logastatin, Sildenafil, because they are metabolized through other isoenzyme cytochrome P 450. It is necessary to adjust the doses of drugs and control plasma concentrations.
Contraindicated reception of clarithromycin during the use of midazolam, triazolam, alprazolam, pymoside, thermal, cispanis, asthymizola, ergotamine and other breathing alkaloids.
Possible medicinal interactionsthat should be taken into account:
- other macrolide antibiotics, clindamycin, lincomycin: the development of cross-resistance is possible;
- derivatives (ergotamine, dihydroergotamine): There is a risk of developing acute ergotamine intoxication (manifested by symptoms such as perverted sensitivity, heavy peripheral vasospasm, limb ischemia and other tissues, including the central nervous system);
- gMG-CoA-reductase inhibitors (Simvastatin, Lovastatin): rare cases of rhabdomyolysis are described;
- medicinal productswhich are primarily metabolized by CYP3A with the inhibitors of CYP3A4 inhibitors (itraconazole): a mutual increase in concentrations is possible, as a result of which strengthening or prolongation of therapeutic and side effects may be marked;
- triazoles: It is possible to reduce its clearance and increase it pharmacological effects (including the development of sleepiness and confusion);
- digoxin: It may increase its plasma concentration (control is required to avoid digitalistic intoxication and the development of potentially lethal arrhythmias);
- atazanavir, ritonavir and other protease inhibitors: a mutual increase in plasma concentrations is noted (clarithromycin in this case can not be used in daily doses of more than 1000 mg);
- fluconazole in a dose of 200 mg (in case of simultaneous use of clarithromycin in a daily dose of 1000 mg): an increase in AUC and an equilibrium concentration of clarithromycin by 18 and 33%, respectively (dose correction is not required);
- etravin: The concentration of clarithromycin is reduced, however, the content of its active metabolite increases;
- verapamil: There is a possibility of decline arterial pressure, development of bradyrithmy and lactic acid acidose;
- oral hypoglycemic drugs, insulin: the risk of hypoglycemia has appears (it is necessary to control blood glucose);
- some drugs that can cause a decrease in glucose concentration (pioglitazone, Rosiglitazone, repaglinide, nateglinide): It is possible to inhibit the Cyp3a Clarithromycin inhibitory, which leads to hypoglycemia (careful glucose control is required);
- inductors of cytochrome p 450 (rifabutin, rifampicin, rifapentine, nevirapin, efavirenz): the plasma concentration of clarithromycin is reduced, it decreases therapeutic effect, the content of active metabolite 14 (R) is hydroxiclaritromycin;
- zidovudine: It is possible to reduce its equilibrium level (the correction of doses of drugs is required);
- asthemisol, Terfenadine, Pimozide, Cezaprid: Their plasma concentrations may increase, which leads to the elongation of the Qt-interval and the development of heart arrhythmias (including flickering or vasting, fibrillation, ventricular paroxysmal tachycardia, polymorphic ventricular tachycardia by the type "Pirouette"). Such interactions are also possible when using drugs that are metabolized by the system of the cytochrome p 450 system (for example, valproic acid, theophylline, phenyotine) (control of the concentrations of drugs in the blood and monitoring of the electrocardiogram);
- savinavir in soft gelatin capsules at 1200 mg 3 times a day (simultaneously with claroomycin in a daily dose of 1000 mg): an increase in AUC and an equilibrium concentration of saquinavir, respectively, by 177 and 187%, clarithromycin - by 40% (with short-term therapy with drugs in these doses You do not need to adjust the latter);
- tolterodin: its effect in patients with low activity of the CYP2D6 isoenzyme (dose reduction may be required);
- colchicine (substrate for CYP3A and P-glycoprotein): It is possible to strengthen its action (careful observation of patients is required for the development of symptoms toxic action Colchicine).
Amoxicillin
- phenylButazone, oxyphenbutayon, allopurinol, non-steroidal anti-inflammatory drugs, diuretics and other drugs that block the channel secretion: the plasma concentration of amoxicillin is reduced;
- bacteriostatic preparations (chloramphenicol, sulfonamides, lincoosamides, macrolides, tetracyclines): An antagonistic effect is noted;
- bactericidal antibiotics (including rifampicin, vancomycin, aminoglycosides and cephalosporins): a synergistic action is noted;
- methotrexate: its clearance decreases and toxicity increases (careful control of plasma concentration is required);
- samples: reduced by the removal of amoxicilline kidneys, the concentration in plasma and bile increases;
- digoxin: It is possible to increase its suction time (dose correction may be required);
- metronidazole: There are such side effects as disorders of digestion, nausea, constipation, diarrhea, pain in epigastria, anorexia, vomiting, in rare cases - impaired hemopower, jaundice, interstitial jade;
- estrogens, progesterons: their plasma concentration is reduced, which is why the contraceptive effect is possible (additional non-correlative contraceptive methods must be used);
- indirect anticoagulants: their action is enhanced, the blood coagulation time is extended (dose correction may be required);
- allopurinol: The risk of skin rash increases;
- ascorbic acid: amoxicillin absorption is enhanced;
- glucosamine, aminoglycosides, laxatives, antacids, food: slows down and reduced absorption of amoxicillin.
Lansoprazole
With the simultaneous use of other drugs, it should be borne in mind that Lansoprazol:
- compatible with warfarin, propranolol, indomethacin, prednisolone, phenythine, diazepam, ibuprofen, oral contraceptives;
- slows down the removal of drugs that are metabolized in the liver by microsomal oxidation (including indirect anticoagulants, diazepam and phenytoin);
- prevents the suction of iron salts, itraconazole, digoxin, ampicillin, ketoconazole;
- reduces theophylline clearance by 10%;
- slows down the pH-dependent absorption of drugs from a group of weak acids;
- accelerates the absorption of drugs from the base group;
- slows down the suction of cyanocobalamin;
- increases the plasma concentration of the tacrolimus (the substrate of the CYP3A4 and P-glycoprotein isoenzyme) to 81% (plasma concentration control is required);
- significantly reduces atazanavir with MAH and AUC (contraindicated combination);
- increases the risk of atorvastatin's myotoxicity, Lovastatin, Simvastatin (patients should be under careful medical supervision).
The influence of other drugs on Lansoprazol:
- antacids reduce and slow down absorption (1-2-hour intervals between receptions should be observed;
- fluvoxamine (inhibitor of the CYP2C19 isoenzyzerlet) increases the plasma concentration;
- john's wortradly, rifampicin (inductors of CYP3A4 and CYP2C19 isoenzymes) can significantly reduce plasma concentration;
- sukralfat reduces bioavailability by 30% (intervals of 30-40 minutes should be observed between the techniques);
- ritonavir (the substrate and the CYP2C19 inhibitor) can be both increased and reduced AUC (a dose correction of Lansoprazole and the monitoring of therapeutic and possible side effects is required);
- imatinib increases the risk of developing side effects, especially in patients with severe allergic reactions in history (due to potential interaction through CYP3A4).
With the simultaneous use of antiretroviral drugs (Atazanavira, Indinavir, Nelfinavira), ketoconazole, cefpodoxy, kotokonazole, itraconazole, ampicillin, cefurocama must be controlled by their effects and symptoms of resistance.
With a combined appointment of clopidogrel, the risk of myocardial infarction increases, as well as hospitalization about stroke, unstable angina, cardiac attack, repeated revascularization, therefore it is recommended to avoid its combination with Lansoprazole. With the absolute need for joint use of drugs, careful monitoring of patients should be ensured.
It is not recommended to assign Lansoprazole with HIV-infected patients receiving antiretroviral drugs. If simultaneous use is necessary, the intervals between receptions of 12 hours should be observed, while the dose of lansoprazole should not exceed 30 mg.
Analogs
Analogues of Lancide Whale are pythobakt, pythobact AM, HELITRICS.
Terms and conditions of storage
Store at a temperature not higher than 25 ° C in a dry, light-protected place inaccessible for children.
Shelf life - 3 years.
Medical Instructions
drug
GDU A PLUSWHALE
Tradename
GDU Plus Kit.
International unpatient name
Dosage form
Capsules and tablets covered with film shell
Structure
Omeprazole
One capsule contains
active substance- omeprazole ** 20 mg,
structure coatings granules: Mannitol, hydroxypropylmethyl cellulose (NRMS), sodium laurylsulfate, dynatory hydro phosphate, sucrose, titanium Dioxide E171, calcium carbonate, diethyl phthalate, twin-80, sodium hydroxide, Drug coating L-30D (acryoocate-L100, calcium phosphate main, lactose).
the composition of the shell capsule: Diamond Blue E133, Titanium Dioxide E171, Methylpagidroxybenzoate, Propyl Paulroxibenzoate, Sodium Laurilsulfate, Gelatin.
ink composition:ethanol, 2-propanol, shellac, titanium Dioxide E171, ammonia solution, polysorbat-80.
Tinidazole.
One tablet contains
active substance -tinidazole 500 mg,
excipients:cellulose microcrystalline, corn starch, chinoline yellow E104, sodium croscarmellos, silicon colloidal dioxide, magnesium stearate, peeled talc.
the composition of the shell:hydroxypropylmethylcellulose, ethylene cellulose, PEG-600, propylene glycol, titanium Dioxide E171, quinoline yellow E104.
Clarithromycin
One tablet contains
active substance -clarithromycin 500 mg
excipients:cellulose microcrystalline, poverant, stearic acid, sodium croscarmellos, silicon colloidal dioxide, talc peeled, magnesium stearate.
the composition of the shell:hydroxypropylmethylcellulose, titanium Dioxide E171, ethylcellulose, PEG -600, propylene glycol.
Description
Omeprazole
Solid, transparent gelatin capsules of oval shape with a housing and a blue-sized cover No. 2, with the inscription "Plethico / plethico" applied with white paint on the housing and a lid.
The contents of the capsule are almost whitegranulated.
Tinidazole.
Tablets of oval shape with a biconnecting surface, coated film heater, with risky on one side.
Clarithromycin
Oval shape tablets with a bico surface, covered with film shell from white to grayish-white, with risky on one side.
Pharmacotherapeutic group
Anti-sized drugs and preparations for the treatment of gasttroezophageal reflux. Combinations of drugs for Eradication Helicobacter Pylori.
A02BD PBX code
Pharmacological properties
Pharmacineika
Omeprazole
The antisecretory effect of omeprazole occurs within 1 hour, with the achievement of the maximum effect for 2 hours. The secretion suppression is 50% of the maximum in 24 hours, and the duration of action is 72 hours. Suction occurs quickly, with the achievement of peak plasma levels after 0.5-3.5 hours. Binding of plasma proteins is approximately 95%. Omeprazole is quickly and fully metabolized. Metabolites are inactive and derived mainly with urine and to a lesser extent through bile.
Clarithromycin
Clarithromycin is quickly absorbed from the gastrointestinal tract and is subjected to preserved metabolism; Absolute bioavailability is approximately 55%. Peak plasma concentrations are achieved 2-3 hours after receiving the drug inside. Sustainable concentrations are achieved for 3 days and are approximately 3 to 4 μg / ml, when taking a dose of 500 mg every 8 - 12 hours. Pharmacokinetics of clarithromycin is nonlinear and depends on the dose.
Clarithromycin and 14-hydroxyclarythromycin are distributed in all tissues of the body. Its concentration in tissues is higher than in serum, partly due to intracellular absorption. Clarithromycin is found in B. breast milk. The drug is extensively metabolized in the liver, and is excreted breened with feces. When appropriate 500 mg of the drug every 12 hours, the selection of clarithromycin with urine is about 30%.
14-hydroxyclarithromycin, like other metabolites, which account for 10-15% of the dose, is also displayed with urine. As reported, after taking the drug for 500 mg twice a day, the final half-life of clarithromycin is from 5 to 7 hours.
Tinidazole.
Tinidazole is quickly and almost completely absorbed after taking inside, the plasma half-life is 12-14 hours and, as a rule, peak plasma concentrations make up about 40 μg / ml and are reached 2 hours after one-time 2 dose, and reduced to 10 μg / ml per 24 hours and up to 2.5 μg / ml at 48 hours; The concentrations of over 8 μg / ml are maintained at a daily reception at a dose of 1. The plasma half-life of Tinidazole is 12-14 hours.
Tinidazole is distributed throughout the body: in bile, breast milk, spinal fluid, saliva; And most of other tissues of the body achieve the concentration of the drug like plasma concentrations. The drug penetrates well through a placental barrier. Only 12% of the drug binds to plasma proteins. The plasma detects active hydroxy metabolites of the drug. The drug is unchanged and its metabolites are removed by urine and, to a lesser extent with feces.
Pharmacodynamics
Combined therapy, including omeprazole, Clarithromycin and Tinidazole, allows to achieve a high percentage of eradication Helicobacter pylori.(60-70%). Omeprazole depresses the secretion of gastric acid due to the specific inhibition of H + -K + -atfase - the enzyme in the membranes of the stomach mucosa membranes. Reduces basal and stimulated secretion regardless of the nature of the stimulus. After a single reception of the drug inside, the effect of omeprazole occurs during the first hour and continues for 24 hours, the maximum effect is achieved after 2 hours. After the discretion of the drug, secretory activity is completely restored after 3-5 days.
Clarithromycin is an antibiotic from the group of macrolides, the semi-synthetic derivative of erythromycin A. has an antimicrobial effect, which is associated with the suppression of protein synthesis by interacting with the 50s ribosomal subunit of the microbial cell. Effective with respect to a large number of gram-positive, gram-negative aerobic and anaerobic microorganisms, including N. pylori.. Metabolite 14-hydroxyclarythromycin formed in the body also has a pronounced antimicrobial activity.
Tinidazole Antiprotozoic preparation with antimicrobial action. The mechanism of action of the drug is associated with the oppression of synthesis and a violation of the structure of DNA of sensitive microorganisms. Active in relation to Trichomonas Vaginalis, Entamoeba Hystolitica, Lamblia. It has a bactericidal effect on Bacteroides SRR. (including Bacteroides Fragilis, Bacteroides Melaninogenicus), Peptostreptococcus SPP., Peptococcus SPP., Veilonella SPP.
Indications for use
Eradication of Helicobacter Pylori in infected patients with ulcerative disease Stomach and 12 pans, chronic gastritis (as part of combination therapy)
Method of application and dose
In the morning, 1 capsule of omeprazole is taken to meals and 1 tablet of clarithromycin and tinidazole after meals, in the evening they repeat the reception of these drugs in the same mode. Tablets do not chew. The total duration of therapy is 7 days.
Side effects
Possible
Headache, dizziness, excitation, drowsiness, insomnia, paresthesia, depression, holucination, enzeloofapathy
Dry mouth, violation of taste, nausea, vomiting, abdominal pain, stomatitis, hepatitis, violation of the liver function
Hardwriter, skin rash, itching, photosenswebelization, muscle weakness, Malgy, multiform exudative erythema, alopecia angioederate swelling, bronchospasm anaphylactic shock, fever
Back pain
Gynecomastia
Seldom
- leukopenia, tramshotopenia, agranulocytosis
In isolated cases
ATPROFIC GASTRITE IN THE BIOPS OF THE STROT OF PATIENTS, for a long time received omeprazole
Clarithromycin
Allergic reactions (urticaria, anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necroliz)
Arthralgia, Malgy
Glossite, Stomatitis, anorexia, vomiting, discoloration of the tongue, change in coloring of teeth, pancreatitis, hepatocellular, cholestatic hepatitis with jaundice or without jaundice
Dizziness, anxiety, behavioral changes, confusion, convulsions, depersonalization, disorientation, hallucinations, insomnia, "nightmarish" dreams, paresthesia, psychosis, rings in the ears, amendment of sense of smell, the perversion of taste or loss of taste
The elongation of the Qt interval, ventricular arrhythmia, including ventricular tachycardia
Interstitial nephritis
Collycinic intoxication
Thrombocytopenia, leukopenia, neutropenia, enhancement of liver enzymes, hypoglycemia, increase in prothrombin time, high whey creatinine
Tinidazole.
Metal / Gorky taste in the mouth, in addition, the loss of appetite, dyspepsia, a sense of discomfort in the epigastric area, constipation, nausea, vomiting, headache, dizziness and malaise.
Dizziness, Ataxia, insomnia, drowsiness
Discoloration language, stomatitis, diarrhea
Hardwriter, itching, rash, redness, sweating, fever, burning, thirst, hasslement, swelling quinque
Reinforced heartbeat
Transient neutropenia / leukopenia
Enhanced vaginal discharge, candidal stomatitis, liver disorders, including raising the level of transaminase, arthralgia, Malgia.
Contraindications
Well-known increased sensitivity to omeprazole, clarithromycin, tinidazole, other macrolides or any of the components of the drug
Joint reception of omeprazole with atazanavir, cisapride, pimoside, thermal, asthymizola (because it is possible to lengthen the Qt interval, cardiac arrhythmia, including ventricular tachycardia, ventricular fibrillation, vulgarity flicker)
The joint use of clarithromycin with ergotamine or dihydroeergotamine (with co-administration of clarithromycin with ergotamine or dihydroergotamin, ergotamic intoxication was noted, manifested by spasms of vessels and ischemia limbs and other tissues, including the central nervous system)
Infectious mononucleosis and leukemoid reactions
Heavy liver and renal failure with creatinine clearance less than 30 ml / min
Malignation of stomach ulcers
Children's and teenage age up to 18 years
Pregnancy and lactation period
Medicinal interactions
Omeprazole
Omeprazole can extend the period of removal of diazepam, warfarin and phenytoin, drugs metabolized by oxidation in the liver.
As a result of pronounced and long oppression gastric secretion The drug may disrupt the suction of such drugs as ketoconazole, ampicillin and iron salts.
Clarithromycin
The joint appointment of clarithromycin with such drugs as clarid, pimozide or thermopenadine can lead to the development of heart arrhythmias (the elongation of the Qt interval, ventricular tachycardia, ventricular fibrillation, bidirectional ventricular tachycardia), and the oppression of the liver metabolism of these drugs.
With joint appointment with theophylline, it is possible to increase the plasma concentrations of theophylline.
When prescribing single doses of clarithromycin and carbamazepine, an increase in plasma concentrations of carbamazepine was noted.
With simultaneous purpose with Zidovudine, HIV-infected adult patients a decrease in plasma concentrations of Zidovudine was noted.
A dose correction may be required when clarithromycin and ritonavir in patients with impaired kidney function may be required.
The joint appointment of clarithromycin and oral anticoagulants can lead to an increase in the action of oral anticoagulants.
There was an increase in plasma concentrations of digoxin in patients receiving at the same time clarithromycin and digoxin.
With the joint appointment of erythromycin or clarithromycin with ergotamine or dihydroergotamin, an acute ergotamic toxicity was developed, characterized by a pronounced spasm of peripheral vessels and dysteste.
Clarithromycin causes an increase in the concentrations of HMG-CO inhibitors of reductase (for example, lovastatin or simvastatin), due to the inhibition of cytochrome P450.
Colchicine:colchicine is a substrate for Cyp3a and p-glycoprotein. Clarithromycin and other macrolides are CYP3A and P-glycoprotein inhibitors. With the joint appointment of colchicine and clarithromycin, the inhibition of p-glycoprotein and / or CYP3A can lead to an increase in the action of colchicine. Patients should be carefully observed in order to identify the symptoms of the toxic action of colchicine .
Ranitidine, bismuth citrate:the simultaneous administration of clarithromycin with ranitidine, bismuth citrate leads to an increase in the concentration of ranitidine in a plasma by 57%, an increase in the concentration in the platter of bismuth citrate by 48% and an increase in the concentration of 14-hydroxyclarithromycin in plasma by 31%. These effects are clinically insignificant.
Joint appointment with drugs, metabolized with the participation of the cytochrome P450 system (carbamazepine, cyclosporine, tacolimus, hexobarbital, phenytoin, alphantanyl, dyspeyramide, lovastatin, bromocriptine, valproate, rifabutin and asthysis) can lead to an increase in plasma levels of these drugs.
Tinidazole.
Tinidazole violates metabolism or elimination next drugs: Warfarin, Phenitoin, Lithium, Cyclosporine and Formuracyl.
With joint admission with alcoholic beverages, the Tinidazole is manifested by a disulfira-like action.
special instructions
Application in children
The effectiveness and safety of the drug GDU TM Whale for children is not established.
Before starting therapy, it is necessary to eliminate the presence of a malignant process (especially with a stomach ulcer), because Treatment, masking symptoms, can delay the setting of the correct diagnosis.
Clostridium Professional Diarrhea Diffix (CDAD) was noted when using almost all antibacterial drugs, including clarithromycin, and maybe by severity from moderate diarrhea to fatal colitis . Treatment with antibacterial agents leads to a change in normal thick intestinal flora and can lead to enhanced growth of clostridium. C..difficile Repair toxins A and B, which contribute to the development of CDAD. Hypertoxin produced C..difficile, increases morbidity and mortality, since this infection is immune to antibacterial therapy and can lead to a kolactomy. It is necessary to carefully collect anamnesis of the disease, since CDAD is known to develop more than two months later after applying antibacterial drugs.
If a "pseudomambranous colitis" is suspected or installed, it is necessary to cancel the antibiotic and begin the appropriate treatment.
Reported on colchicine intoxication in the joint use of clarithromycin and colchicine, especially in elderly and patients with renal failure.
Co-use of clarithromycin with cisapride or pimisid - contraindicated.
Clarithromycin in combination with ranitidine, bismuth citrate should not be used in patients with acute porphyrene in history.
Patients with impaired kidney function
The dose of the drug GDU TM WHO in patients with severe renal failure (KK is no more than 30ml / min). Special caution should be taken when prescribing the drug of this category of patients.
Application in patients with impaired liver function
Omeprazole is metabolized in the liver and in patients with disturbed liver function, the half-life of the drug is extended, so the dosage of the drug in such patients should be reduced.
Elderly patients
It may be necessary to reduce the dose of the GDU TM Whale in elderly patients, especially when the kidney function is severe. Special caution should be taken when prescribing the drug of this category of patients.
Effect of drug on the ability to control vehicles and potentially hazardous mechanisms
Considering side effects medicinal product, care must be taken when managing motor transport and other potentially hazardous mechanisms.
Overdose
O.meprazole.
There are single messages about taking the drug in doses of 320 to 900 mg (which is 16-45 times the recommended therapeutic dose). Symptoms: The confusion of consciousness, lethargy, boldness, tachycardia, nausea, increased sweating, redness of the face, headaches, dryness of the mucous membrane. These symptoms were transient, without any severe consequences for the body.
Treatment: There is no specific antidote. Omeprazole is extensively associated with proteins and therefore poorly excreted during hemodialysis. In case of overdose, symptomatic and supporting treatment should be prescribed.
Tinidazole.
Cases of overdose are not described.
Symptoms
Treatmentsymptomatic and supportive. There is no specific antidote. Stomach washing can be shown. Tinidazole is quickly excreted from the blood using dialysis.
Clarithromycin
Symptoms: Allergic reactions and gastrointestinal symptoms.
Treatment: Stomaching and symptomatic therapy. Hemodialysis and peritoneal dialysis are ineffective.
Form release and packaging
2 Capsules omeprazole, 2 Tinidazole tablets and 2 clarithromycin tablets are packaged in contour bale-free packaging from aluminum foil.
7 contour packages, together with the instructions for medical use in public and Russian, are placed in a cardboard pack.
Storage conditions
Store in a dry, light-protected place at a temperature not higher than 25 ° C.
Keep out of the reach of children!
Storage term
Do not apply after the expiration date specified on the package.
Conditions of vacation from pharmacies
On prescription
Manufacturer
A.B. Road, Mangliament - 453 771, Indore (MP), India
37 / 37A, Industrial Evail, Poly Rogue, Indore (MP), 452 015, India
Owner of the registration certificate
"Plethico Pharmaceuticals Ltd / Plotchiko Pharmal Ltd."
The address of the organization hosting on the territory of the Republic of Kazakhstan complaints from consumers for product quality (product)
LLP "RELLS LTD",
100009 Karaganda, ul. Ermekova, 110/2.
Tel .: / 7212/48 16 44, 43 15 34, 43-15-63,48-17-67; Tel. / Fax: / 7212/48 17 44; E-mail [Email Protected] , [Email Protected]
Are you a hospital leaf due to back pain?
How often do you encounter a back pain?
Can you carry pain without receiving painful agents?
Learn more as quickly as possible to cope with the back pain
Laboratory indicators: very rarely - increase in cholesterol and triglycerides, hyponatremia.
Method of preparation or application:
Inside. Take 500 mg (1 tablet) of clarithromycin, 1000 mg of amoxicillin (2 capsules) and 30 mg of lansoprazole (1 capsule), twice a day in the morning and in the evening before meals. Tablets and capsules can not be broken and chewed, they should be swallowed entirely. The duration of treatment is 7 days, if necessary, can be increased to 14 days. Each blister of the drug Lancide whale contains two tablets of clarithromycin (500 mg), four amoxicillin capsules (500 mg) and 2 lanceprazole capsules (30 mg) and is designed for one day of treatment. One package contains 7 blisters and is designed for one treatment.Leave order:
In the form of a prescription form 107-1 / y
Storage conditions
:Store in a dry, protected from light, at a temperature not higher than 25 ° C.
Is a drug. Consultation is needed.
Antibiotic Clasid contains an active ingredient, as well as additional components: sodium calcium alginate, sodium alginate, lactose, anhydrous lemonic acid hydrophosphate, stearic acid, poveline KZO, magnesium stearate.
Form release
Clastide 500 mg and 250 mg is produced in the form of tablets that cover the sheath of yellow. Tablets have an oval shape, in the cut, there are two layers: the yellow film and the kernel of the whitish color. In a blister made of foil, there may be 7, 10 or 14 tablets, 1, 2 or 3 such blisters are packed in a cardboard box.
pharmachologic effect
Active substance clarithromycin Included in the group of macrolides, semi-synthetic. Antibacterial effects produces by suppressing the synthesis of protein bacteria. The consistency of the tablet is such that the active ingredient is released gradually, in the course of passing the drug on the gastrointestinal tract. Clarithromycin Active with respect to isolated and standard bacteria cultures. The high effect is noted when applying means for treating the disease of legionnaires, pneumonia of mycoplasma etiology. Gram-negative bacteria do not show sensitivity to clarithromycin .
The active ingredient actively acts as an antibacterial agent against streptococci Group A. , pneumococcus , Golden staphilococcus , trap microorganisms hemophilic infection , liseriosis , pneumonia , pneumochlamodoza , leprosy , , face , aport.
Those causative agents who do not demonstrate sensitivity to and Methicillin Also resistant to influence clarithromycina .
Also noted positive impact clarithromycina in relation to the following microorganism (efficiency and safety is not confirmed in the process clinical studies): Green streptococcus, peptococcus, streptococci groups B, C, F, G; Pastellosis pathogens of birds, toxicoinfection of a person, , borrelliosis, enterocolitis.
In the process of metabolism clarithromycina In the body allocated active 14-hydroxyclarithromycin, manifesting microbiological activity. Metabolism occurs in the liver of a person. If a person has taken the drug regularly, there was no amplification of activity of its influence.
Pharmacokinetics and pharmacodynamics
Substance clarithromycin binds to blood proteins well. The largest concentration of the drug is determined for 6 hours. The greater dose of the drug accepted the patient, the longer period of time it is excreted from the body. The amount of metabolite (14-hydroxyclarithromycin) does not increase in parallel with the increase in the dose of clarithromycin. The greater the adopted dose of Clazide, the less than 14-hydroxyclarithromycin is formed in the body.
From the body, the medicine is excreted through the kidneys and intestines (respectively, 40% and 30% dose). After oral administration of clarithromycin, and its metabolite is distributed over the tissues and fluids of the body, in tissues, as a rule, is two times more than the drug compared with serum.
No dosage correction is required for liver disease. With kidney diseases, the removal period clarithromycina It increases from the body. Also, the period of removal of the drug increases in the older people.
Indications for use
The use of the drug Clasid is shown in the following diseases and states:
- infectious diseases of the respiratory tract, lower departments ( pneumonia , and etc.);
- infectious diseases of the respiratory tract, upper departments (at, etc.);
- infectious lesions of soft tissues, skin cover ( follyculite , rygin and etc.);
- mycobacterial infections that provoked Mycobacterium Intracellulare and Mycobacterium Avium;
- infections provoked by Mycobacterium Fortuitum, Mycobacterium Chelonae, Mycobacterium Kansasii.
Reception is also practiced to prevent infection by the Mycobacterium AVIUM complex (Mac). It is assigned to reduce the frequency of manifestations of the recurrence of duodenal ulcers.
Contraindications
It is impossible to take an antibiotic in the following cases:
- with the body to the means of the macrolide group;
- for porphyry ;
- during pregnancy and breastfeeding ;
- children up to 3 years of age.
Caution The remedy is prescribed in disabilities of the kidneys and liver.
It is impossible to take at one time clarithromycin and drugs such: D.igidroergotamine , Pimozide , E.rgotamine , BUTstemisol .
Side effects
If the Clasid is introduced in / B or oral administration is carried out, the manifestation of a number is possible side phenomena. If there are such effects after I / in administration or intake, the tablets need to report this to a specialist. The following manifestations are possible:
- CNS functions : Changes in taste quality ,.
- Digestion system : nausea , stomach ache, .
- Local reactions when introducing a solution : inflammatory processes at the point of administration, phlebitis , Pain during palpation.
- Laboratory indicators : Increase the activity of liver enzymes.
In addition to such side effects, side effects that manifest themselves are less common:
- oral cavity;
- thrombocytopenia , lakeing ;
- hypoglycemia ;
- psyche disorders;
- , causes ;
- malgy ;
- reversible hearing loss;
- ventricular;
- stomatitis , sharp , ;
- violation of the liver function;
- increased creatinine content in the blood.
Clazide application instructions (method and dosage)
Instructions for the use of claside for children and adults involves the reception of the means inside, regardless of food intake.
Adult patients showing 250 mg of clarithromycin twice a day. If treatment is carried out by severe diseases, mycobacterial infections, the dose can be increased to 500 mg twice per day. In most cases, the treatment continues from 5 to 14 days.
If treatment is assigned suspension Clacid The instructions for use should be accurately observed. Suspension is prescribed for treating children, it can be taken regardless of food eating, you can take with milk. To prepare a suspension for use, you need to gradually add water to the label, then shake. In 5 ml of 60 ml suspension contains 125 mg of clarithromycin; In 5 ml of suspension, 100 ml contains 250 mg of clarithromycin. You can store a suspension for two weeks at room temperature.
Before giving an antibiotic Clasid for children, you need to carefully shake the suspension. On a day, it is recommended to apply the dose to children at the rate of 7.5 mg per 1 kg of body weight twice per day. The greatest permissible dose is 500 mg twice a day. Therapy may continue from 5 to 10 days.
Overdose
When receiving very large doses of this drug, patients may have a manifestation of signs of functioning digestive system. It is important to remove the remnants of the drug from the tract as quickly as possible, after which symptomatic treatment is carried out.
Interaction
It is categorically not allowed sharing clarithromycina and drugs Asthemisol , Pimozide , Terfenadin , Cisprid Since, in this case, probably the development of serious side effects. In particular, the manifestation of heart arrhythmias is possible, including ventricular fibrillation, ventricular tachycardia.
With the simultaneous use of clarithromycin and Ergotamine Or probably acute poisoning by means of the Ergotamin Group. In particular, the limb ischemia, vascular spasm, etc. can manifest.
CYP3A inductors induce clarithromycin metabolism. As a result, the concentration of clarithromycin and its effectiveness decreases. If clarithromycin is used at the same time and concentration increases in the plasma Rifabutin And the concentration of clarithromycin decreases.
Preparations Neusarapin , Efavirenz , Rifabutin , Rifapentin Claimed clarithromycin metabolism can accelerate, thereby reducing its plasma concentration and increasing the concentration of its metabolite - 14-parameomycin. As a result, therapeutic efficacy may decrease.
Reduced concentration clarithromycina it is noted with the joint reception with etravirin .
It is necessary to adjust the doses of drugs when clarithromycin and ritonavira .
With simultaneous treatment with claside and oral hypoglycemic drugs or insulin, a pronounced hypoglycemia is possible. The glucose level should be constantly monitored.
With the simultaneous reception of the antibiotic with Quinidine , Dizeciramid. Possible ventricular tachycardia.
Carefully need to take clarithromycin to those who receive medicines - the substrates of the CYP3A isoenzyme, as well as in combination with statins.
The simultaneous treatment with clarithromycin and is contraindicated.
It is important to control the patient's condition when co-treatment and clarithromycin due to the probability of bleeding.
With clarithromycin and There is a need to reduce the dosage of the latest drugs.
Simultaneous treatment with antibiotics and carbamazepine leads to an increase in the concentration of these drugs in the bloodstream.
When using clarithromycin and triazolam Probably influence on CNS, as a result, drowsiness and confusion of consciousness develop.
It is impossible at one time to take clarithromycin and colchicine people who have breakdown or kidney functions.
With simultaneous treatment with clarithromycin and the latter action is enhanced. Permanent control of the level of digoxin in serum is needed.
There is a bidirectional effect of drugs with simultaneous reception of clarithromycin and atazanavira , as well as clarithromycin and itraconazole, clarithromycin and savicinavira .
When treating simultaneously with an antibiotic and, Diltiazem the likelihood of the development of arterial hypotension increases.
Terms of sale
In pharmacies, the antibiotic is sold according to the recipe.
Storage conditions
You need to take care of the antibiotic from the access of children, it should be stored at a temperature not higher than 30 ° C.
Shelf life
The shelf life of the antibiotic is 3 years.
special instructions
People with liver disease may have a change in serum enzyme indicators, from which the tablets must be prescribed carefully.
Caution is prescribed to people in parallel with other medicines that are metabolized by the liver.
With prolonged treatment with antibiotics, the formation of colonies with a large number of insensitive fungi and bacteria.
For chronic diseases The liver must regularly monitor serum enzymes.
Perhaps manifestation pseudomambranous colitis When treating an antibiotic. Also possible change normal microflora intestines.
Caution should be prescribed to people, severe heart failure, bradycardia, hypomantee. It is necessary to constantly control the ECG, determining the increase in the Qt interval.
It is possible to strengthen the symptoms In people who accept Clarithromycin.
In the powder for the preparation of suspension, Clasid contains sucrose, which should be taken into account by people suffering.
In the treatment of clarithromycin, it is necessary to gently control the transport and perform actions requiring high concentration attention.
Synonyms
Clarithromycin
Kaccide analogues
Coincidences on the ATX 4 level code:Kaccide analogs - funds that are included in the same group and have a similar clacide active substance. These are medicines, Clarecis , Binoclaw , Claricit , Coldrosyne , Clarithromycin , Clarein et al. Replace the tool only after the approval of the doctor, since each of these drugs exists certain features of use and side effects.
The price of analogs can be both higher and lower. Difference Clocid and Clastide CP that last Preparation It is a cure for prolonged action, that is, the active ingredient is released more slowly.
With alcohol
As the instruction, claside and alcohol are incompatible. If, in the treatment of this antibiotic, the patient consumes alcohol, the risk of photography of side effects increases sharply, as the toxicity of the drug is strongly increasing.
Clasid for children
Clasid for children can be applied from a three-year age. In most cases, the Suspension of Clasid is prescribed to children. Reviews for children indicate that this drug is quite effective. At the same time, the price of the suspension is high enough. The dosage for children is as follows: 7.5 mg of money per 1 kg of child weight twice a day. Most daily dose - 500 mg.
Children who have already been 12 years old are appointed 250 mg (tablets) twice a day. There is evidence that children carry claside easier than other antibiotics. Therefore, the drug is often prescribed when , bronchitis , pneumonia et al. However, we should not forget that side effects still take place.
During pregnancy and lactation
There is no accurate information about the safety of the use of the clocide for treatment and nursing mothers. Therefore, the application during the fetus is not practiced.