Hyperandrogenism of ovarian origin. Adrenal hyperandrogenism in women: key aspects of the disease and methods of treatment of the disease. Promotions and special offers
The pathological manifestation in a person of pronounced characteristic features inherent in the opposite sex often provokes adrenal hyperandrogenism (adrenogenital syndrome). With the development of this syndrome in the body, there is increased content androgens (steroid male sex hormones), leading to virilization.
General information
Virilization (masculinization) of adrenal genesis is caused by excessive production of androgen-type hormones by the adrenal glands and leads to external and internal changes that are atypical for the patient's sex. Androgens are necessary in the body of an adult woman, as they are responsible for important transformations of the body during puberty. In particular, they produce the synthesis of estrogen, and also contribute to the strengthening of bone tissue, muscle growth, are involved in the regulation of the liver and kidneys, and the formation of the reproductive system. Androgens are produced primarily by the adrenal glands and female body ovaries, and in the male, respectively, the testicles. A significant excess of the content of these hormones in women can significantly upset the reproductive system and even provoke infertility.
Causes of adrenal hyperandrogenism
 Hormonal imbalance can cause disease.
Hormonal imbalance can cause disease. The main reason for the accumulation of androgens in the body is a congenital defect in the synthesis of enzymes that prevents the conversion of steroids. Most often, the deficiency of C21-hydroxylase, which synthesizes glucocorticoids, acts as such a defect. Moreover, hormonal imbalance it is a consequence of the influence of hyperplasia of the cortical layer of the adrenal glands or tumor-like formations (some types of tumors of the adrenal glands are capable of producing hormones). Congenital adrenal hyperandrogenism is most often diagnosed. However, sometimes there are also cases of the development of hyperandrogenism due to tumors of the adrenal glands that secrete androgens (Itsenko-Cushing's disease).
Pathogenesis
C21-hydroxylase deficiency can be successfully compensated by the adrenal glands for some time and passes into a decompensated phase during stressful hormonal fluctuations that are created by emotional upheavals and changes in the reproductive system (the onset of sexual relations, pregnancy). When the defect in the synthesis of enzymes becomes pronounced, the conversion of androgens into glucocorticoids stops and there is an excessive accumulation of them in the body.
Features of the development of adrenogenital syndrome in women
Adrenogenital syndrome in women leads to serious changes in the functioning of the ovaries and disorders in the reproductive system. According to statistical studies, every fifth woman suffers from hyperandrogenism to some extent with various manifestations. Moreover, age in this case does not matter, the disease manifests itself at any stage. life cycle starting from infancy.
The impact of hyperandrogenism on ovarian function causes the following manifestations:
- inhibition of growth and development of follicles in the early phase of folliculogenesis is manifested by amenorrhea (absence of menstruation for several cycles);
- slowing down the growth and development of the follicle and the egg, which is not capable of ovulation, can manifest itself as anovulation (lack of ovulation) and oligomenorrhea (an increase in the interval between menstruation);
- ovulation with a defective corpus luteum, is expressed in the insufficiency of the luteal phase of the cycle, even with regular menstruation.
Symptoms of adrenal hyperandrogenism
 Hair on the face in women increases with adrenal hyperandrogenism.
Hair on the face in women increases with adrenal hyperandrogenism. Adrenogenital syndrome has primary and secondary manifestations, depending on the phase of the development of the disease and the factors of its occurrence. Indirect signs the presence of adrenal hyperandrogenism in women are frequent colds, tendency to depression, increased fatigue.
The main symptoms of adrenal hyperandrogenism:
- increased growth hairline(limbs, abdomen, mammary glands), up to hirsutism (hair growth on the cheeks);
- baldness with the formation of bald patches (alopecia);
- skin defects (acne, acne, peeling and other inflammations);
- muscle atrophy, osteoporosis.
The secondary symptoms of adrenogenital syndrome are the following manifestations:
- arterial hypertension, manifested in the form of seizures;
- elevated level blood glucose levels (type 2 diabetes);
- rapid weight gain, up to obesity, in need of therapy;
- intermediate type of formation of female genital organs;
- lack of menstruation or significant intervals between menstruation;
- infertility or miscarriage (for a successful pregnancy, a certain amount of female hormones in the body is necessary, the production of which practically stops in the event of hyperandrogenism).
For citation: Pischulin A.A., Karpova E.A. Ovarian hyperandrogenism and metabolic syndrome // BC. 2001. No. 2. S. 93
Endocrinological science Center RAMS, Moscow
WITH the syndrome of ovarian hyperandrogenism of non-tumor origin or hyperandrogenic ovarian dysfunction, previously called Stein-Leventhal syndrome, is currently, according to the WHO classification, better known in the world literature as polycystic ovary syndrome (SPY).
The clinical picture of PCOS is manifested by a chronic anovulatory state of the ovaries or severe hypofunction of the corpus luteum, which leads to a bilateral increase in the size of the ovaries with thickening and sclerosis of the albuginea. These changes are manifested by a violation of the menstrual function - opsomenorrhea, amenorrhea, but the development of metrorrhagia is not excluded. Violations of folliculogenesis lead to the development of anovulatory primary or secondary infertility.
One of the main diagnostic criteria for PCOS is hyperandrogenemia. - an increase in the level of androgenic steroids in the blood (such as testosterone, androstenedione), which leads to the development of hirsutism and other androgen-dependent dermopathies.
Obesity or overweight often accompanies PCOS. Determination of body mass index (BMI) allows you to identify the degree of obesity. Measurement of indicators of waist (WT) and hips (HB) and their ratio indicates the type of obesity (abdominal type of obesity is unfavorable prognostically, in which WT/HB > 0.85).
In addition to the main symptoms of the disease, the clinical picture is largely determined by the general metabolic disorders such as dyslipidemia, impaired carbohydrate metabolism, an increased risk of developing hyperplastic and tumor processes from the genitals. Dyslipidemia is an increase in triglycerides, cholesterol, low density lipoprotein, very low density lipoprotein and a decrease in high density lipoprotein. These disorders lead to the risk of early development of atherosclerotic vascular changes, hypertension and coronary disease hearts.
Carbohydrate metabolism disorders consist in the development of the insulin resistance-hyperinsulinemia complex, which has recently been the main direction in the study of the pathogenetic links in the development of PCOS.
In the 60s, the pathogenesis of PCOS was associated with a primary enzymatic defect in ovarian 19-hydroxylase and/or 3b-dehydrogenase, combining these disorders under the concept of primary polycystic ovaries. However, in the works of subsequent years, it was shown that the aromatase activity of granulosa cells is an FSH-dependent function.
An increased level of luteinizing hormone (LH), the absence of its ovulatory peak, a normal or reduced level of follicle-stimulating hormone (FSH) with an impaired LH/FSH ratio (2.5-3) detected in PCOS suggested a primary violation of gonadotropic regulation of steroidogenesis in the ovarian tissue with the development of a secondary polycystic ovaries.
Until the mid-1980s, it was believed (SSC Yen theory) that the trigger mechanism in the pathogenesis of PCOS is excessive synthesis of androgens by the adrenal glands during the adrenarche period as a result of an altered sensitivity of the adrenal glands to ACTH or excessive stimulation of the adrenal reticular cortex by a non-ACTH-like factor or under the influence of b -endorphins, neurotransmitters, such as dopamine. Upon reaching critical mass body (especially when its norm is exceeded), the peripheral conversion of androgens to estrogens increases, primarily in the liver and adipose tissue. An increase in the level of estrogens, primarily estrone, leads to hypersensitization of gonadotrophs in relation to luliberin (GnRH). At the same time, under the action of estrone, the production of GnRH by the hypothalamus increases, the amplitude and frequency of impulses of its secretion increase, as a result of which the production of LH by the adenohypophysis increases, the LH / FSH ratio is disturbed, and relative FSH deficiency occurs. Strengthening the effect of LH on the ovaries contributes to an increase in the production of androgens by the thecal cells and their hyperplasia. Relatively low level FSH leads to a decrease in the activity of FSH-dependent aromatase, and granulosa cells lose their ability to aromatase androgens into estrogens. Hyperandrogenism prevents the normal growth of follicles and contributes to the formation of their cystic atresia. Lack of growth and maturation of follicles further inhibits FSH secretion. The increased pool of androgens in peripheral tissues is converted to estrone. A vicious circle is closing.
Thus, the result of a violation of the central and peripheral mechanisms of regulation of steroidogenesis is the development of functional ovarian hyperandrogenism in patients with PCOS.
The pathogenesis of PCOS according to S.S.C. Yen is shown in Diagram 1:
Scheme 1.
In the early 80s, a number of authors proposed a new theory of the pathogenesis of polycystic ovary syndrome, different from the theory of S.S.C. Yen. It has been found that PCOS is associated with hyperinsulinemia, and this syndrome is characterized by both reproductive and metabolic disorders.
The relationship between hyperinsulinemia and hyperandrogenism was already pointed out in 1921 by Achard and Thieris. They described hyperandrogenism in an obese woman with type 2 diabetes and called the condition "bearded woman's diabetes."
Later, D. Bargen found that women with PCOS and hyperandrogenism had basal and glucose-stimulated hyperinsulinemia compared with the control group of women of the same weight, which suggested the presence of insulin resistance. A direct relationship between insulin and androgen levels was revealed, and it was suggested that hyperinsulinemia may be the cause of hyperandrogenism.
In 1988, G. Reaven first suggested that IR and compensatory hyperinsulinemia (GI) play a major role in the development of the syndrome of metabolic disorders. He called him "Syndrome X" . Currently, the term "metabolic syndrome" or "insulin resistance syndrome" is most often used.
Hypotheses of the pathogenesis of hyperinsulinemia and hyperandrogenism The mechanism of occurrence of hyperandrogenism and hyperinsulinemia is not fully understood. Theoretically, three interactions are possible: hyperandrogenism (GA) causes GI; GI leads to GA: there is some third factor responsible for both phenomena.
1. The assumption that GA causes GI is based on the following facts. In women who take oral contraceptives containing progestins with "androgenic properties", impaired glucose tolerance is detected. Long-term administration of testosterone to transsexuals is accompanied by the occurrence of IR. It has been shown that androgens affect the composition of muscle tissue by increasing the number of muscle fibers of the second type, which are less sensitive to insulin compared to the fibers of the first type.
2. Most factors are in favor of GI leading to GA. It has been shown that IR persists in patients undergoing subtotal or total ovarian removal, as well as in women who have long-term use of GnRH agonists, when marked androgen suppression was noted. The administration of diazoxide, a drug that suppresses the release of insulin from the pancreas, caused a decrease in testosterone (T) levels and an increase in the level of sex steroid-binding globulin (SSBG) in patients with PCOS, obesity and hyperinsulinemia. Intravenous administration insulin in women with PCOS led to an increase in circulating levels of androstenedione and T. Measures aimed at increasing insulin sensitivity (weight loss, fasting and a low-calorie diet) were accompanied by a decrease in androgen levels. There is evidence that insulin can directly suppress the production of SHBG by the liver, and in conditions of hyperinsulinemia this effect is enhanced. At the same time, it is believed that insulin, and not sex hormones, is the main regulator of SSSH synthesis. A decrease in the level of SSSG leads to an increase in the concentration of free and, therefore, biologically active T (normally 98% of T is in a bound state).
The hypothesis linking GA with hyperinsulinemia does not answer the question of how the ovary maintains insulin sensitivity in an insulin-resistant state of the body. Several possible explanations have been proposed. Since insulin has many functions, it can be assumed that some of them are selectively defective. Organ-specific insulin sensitivity may be observed. But more likely is the assumption that insulin acts on the ovary not only through insulin receptors, but also through receptors for insulin-like growth factors (IGF).
Insulin receptors and IGF-1 receptors have been identified in human ovaries (in ovarian stromal tissue of healthy women, women with PCOS, in follicular tissue and granulosa cells). Insulin can bind to IGF-1 receptors, although with less affinity than its own receptors. However, in HI, as well as in situations where insulin receptors are blocked or deficient, it can be expected that insulin will bind to IGF-1 receptors to a greater extent.
It is possible that the mechanisms of insulin/IGF-1 stimulation of steroidogenesis in the ovary can be divided into non-specific and specific. Nonspecific are the classical action of insulin on the metabolism of glucose, amino acids and DNA synthesis. As a result, the viability of the cell increases and, consequently, the synthesis of hormones increases. Specific mechanisms include direct action insulin/IGF-1 on steroidogenic enzymes, synergism between insulin and LH/FSH, and influence on the number of LH receptors.
Insulin / IGF-1, acting synergistically with FSH, stimulates aromatase activity in granulosa cell culture and thereby increases the synthesis of estradiol. In addition, they lead to an increase in the concentration of LH receptors, enhancing LH-dependent synthesis of androstenedione by theca- and stromal cells.
The increasing concentration of androgens in the ovary under the action of insulin/IGF-1 causes atresia of the follicles, which leads to the gradual elimination of estrogen- and progesterone-producing granulosa cells, followed by hyperplasia of the thecal cells and luteinization of the interstitial tissue of the ovary, which are the site of androgen production. This explains the fact that the stimulation of ovarian steroidogenesis by insulin manifests itself mainly in the form of hyperandrogenism.
It has been suggested that insulin/IGF-1 can stimulate both LH-dependent cytochrome P450c17a activity in the ovaries and ACTH-dependent P450c17a activity in the adrenal glands. This, apparently, explains the frequent combination of ovarian and adrenal forms of hyperandrogenism in patients with PCOS.
A relationship with the S.S.C. theory is also possible. Yen on the involvement of adrenal steroidogenesis in the pathogenesis of PCOS (Figure 2).

Scheme 2. The action of insulin in polycystic ovary syndrome
V. Insler (1993), having studied the levels of insulin, IGF-1, growth hormone and their correlation with the levels of gonadotropins and androgens in women with PCOS, proposed two models for the development of this syndrome. In obese patients, GI causes an excess production of androgens through IGF-1 receptors, which, acting in synergy with LH, cause an increase in the activity of cytochrome P450c17a, the main controlling enzyme in androgen synthesis. In patients with normal body weight, a relative increase in the concentration of growth hormone stimulates excessive production of IGF-1. From this point on, synergism with LH leads to androgen hyperproduction by the same mechanism as in obese patients. An increase in the level of androgens causes a change in the function of the hypothalamic centers, leading to a violation of the secretion of gonadotropins and changes typical for PCOS (Scheme 3).
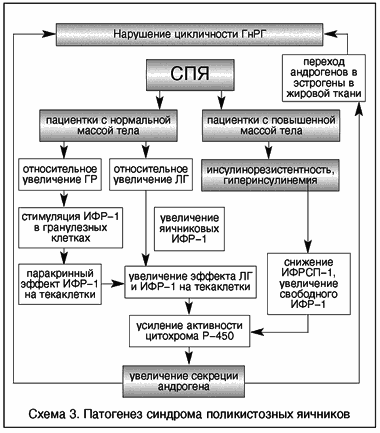
Scheme 3. Pathogenesis of polycystic ovary syndrome
3. However, there are a number of well-known IR conditions that are not associated with GA, such as simple obesity and type 2 DM. To explain why not all obese patients with GI develop hyperandrogenism and PCOS, a hypothesis was put forward about the existence of a genetic predisposition to the stimulating effect of insulin on the synthesis of androgens in the ovary . Apparently, there is a gene or group of genes that makes the ovaries of a woman with PCOS more sensitive to insulin stimulation of androgen production.The molecular mechanisms leading to the development of insulin resistance are not fully understood. However, recent advances in molecular biology have made it possible to determine the structure of the gene encoding the insulin receptor in women with ovarian hyperandrogenism.
Moller and Flier studied the sequence of amino acids in the structure of DNA chains in patients with ovarian hyperandrogenism. They found a substitution of tryptophan for serosine at codon 1200. The researchers suggested that this change disrupts the activation of the tyrosine kinase system in the insulin receptor. Low activity of insulin receptors leads to the development of IR and compensatory GI.
Yoshimasa et al. described another point mutation variant in a patient with hyperandrogenism, insulin resistance, and acanthosis nigricans. They found a substitution of serine for arginine in the tetrameric structure of the insulin receptor. This mutation in the active locus resulted in the impossibility of connecting the a- and b-subunits, as a result of which the functionally active receptor was not synthesized. These studies are only the first attempts to identify a specific genetic etiology of ovarian stromal tecomatosis.
Later, Dunaif A. notes that in polycystic ovary syndrome, IR may be due to impaired autophosphorylation of the insulin receptor b-subunits (iR), the cytoplasmic part of which has tyrosine kinase activity. At the same time, insulin-independent phosphorylation of serine residues (CPOS-ser) increases with suppression of tyrosine kinase activity (a secondary signal transmitter that determines insulin sensitivity to the receptors of the same name). This defect is typical only for PCOS-dependent IR; in other insulin-resistant states (obesity, NIDDM), these changes are not detected.
It cannot be ruled out that some serine phosphorylating factor exists in PCOS-ser. For example, a serine/threonine phosphatase inhibitor is isolated, which, apparently, disrupts uR phosphorylation in PCOS-ser. This compound is similar to the recently isolated membrane glycoprotein PC-1 (inhibitor of insulin receptor tyrosine kinase), but the latter does not increase insulin-independent phosphorylation of serine and R.
Tumor necrosis factor-a (TNF-a) has similar properties: phosphorylation of serine residues IRS-1 (one of the secondary transmitters of the uR signal) under the influence of TNF-a leads to suppression of tyrosine kinase activity of uR.
Moller et al. found that phosphorylation of human P450c17 serine, a key enzyme regulating the biosynthesis of adrenal and ovarian androgens, increased 17,20-lyase activity. Modulation of the enzymatic activity of steroidogenesis by serine phosphorylation has been described for 17b-hydroxysteroid dehydrogenase. If we assume that the same factor (enzyme) phosphorylates insulin receptor serine, causing IR, and P450c17 serine, causing hyperandrogenism, then the relationship between PCOS and IR can be explained. In vitro experiments have shown that protein kinase A (serine/threonine kinase) catalyses serine phosphorylation of insulin receptors (Scheme 4).

Scheme 4. Insulin resistance gene in PCOS
The role of leptin in PCOSRecently, a number of studies have been carried out on biological role leptin, the results of which are encouraging. As a protein hormone, leptin influences feeding behavior and has a permissive effect on the initiation of puberty in animals. The role of this hormone in the regulation of metabolism and reproductive function in humans, unfortunately, has not been fully elucidated. For this reason, data on the level of leptin in ovarian hyperandrogenism in combination with insulin resistance and ideas about its role in the development of these changes are very contradictory.
Recently, a number of studies have been conducted on the biological role of leptin, the results of which are encouraging. As a protein hormone, leptin influences feeding behavior and has a permissive effect on the initiation of puberty in animals. The role of this hormone in the regulation of metabolism and reproductive function in humans, unfortunately, has not been fully elucidated. For this reason, data on the level of leptin in ovarian hyperandrogenism in combination with insulin resistance and ideas about its role in the development of these changes are very contradictory.Thus, according to the results of a study conducted by Brzechffa et al. (1996), a significant proportion of women in the PCOS population have leptin levels higher than expected based on their BMI, free testosterone, and insulin sensitivity. On the other hand, recent work in this area has not shown significant differences in leptin levels in the study groups with PCOS and in the control groups. In addition, it was found that the content of leptin is not affected by the basal level of insulin, the content of gonadotropins and sex steroids. However, Zachow and Magffin (1997), taking into account the presence of leptin receptor mRNA in ovarian tissue, demonstrated a direct effect of this hormone on rat granulosa cell steroidogenesis in vitro. At the same time, a dose-dependent inhibitory effect of leptin on IGF-1 was shown, potentiated by an increase in FSH-stimulated E 2 synthesis by granulosa cells. These data support the hypothesis that increased leptin levels in obese individuals may counteract maturation. dominant follicle and ovulation. Very interesting are the data of Spicer and Franciso (1997), indicating that leptin in increasing concentrations (10-300 ng/ml) inhibits insulin-dependent production of E 2 and progesterone in granulosa cell culture. This effect is due to the presence of specific binding sites for leptin. By analogy with this, it can be assumed that high level leptin can reduce the sensitivity of other target tissues to the action of endogenous insulin, leading to the development of IR in obesity.
Diagnosis Diagnosis of ovarian hyperandrogenism syndrome in typical clinical picture presents no difficulty. First of all, this is a violation of menstrual function by the type of oligo-, opso- or amenorrhea, anovulation and the primary or secondary infertility caused by it, hirsutism, acne, 40% of patients have obesity of varying severity. A gynecological examination reveals a bilateral increase in the size of the ovaries, often against the background of a hypoplastic uterus.
An important place in the diagnosis of PCOS is occupied by hormonal research methods. aimed at identifying hyperandrogenism, its source and determining the level of gonadotropic hormones: LH and FSH. In patients with PCOS, there is often a predominance of the level of LH over FSH, their ratio is disturbed and increased (more than 2.5-3). The level of prolactin is normal, although some increase is observed in 30% of patients.
The level of urinary excretion of total 17-CS in PCOS varies widely and is not very informative. Determination of 17-KS fractions (DGA, 11-oxidized ketosteroids, androsterone, etiocholanolone) also does not provide identification of the localization of the source of hyperandrogenism. Confirmation of the ovarian source of hyperandrogenism is an increase in the level of androstenedione (A) and testosterone (T) in the blood and an increase in the A / T ratio. The adrenal genesis of hyperandrogenism is confirmed by an increase in the level of dehydroepiandrosterone (DHA) and its sulfate (DHA-C) and 17-hydroxyprogesterone (17-OH-P) in the blood. To clarify the localization of the source of hyperandrogenism, various functional tests have been proposed, the most common of which is the test with dexamethasone, synacthen depot.
Taking into account the discovery of new pathogenetic links in the development of PCOS, to assess the state of carbohydrate metabolism, it is necessary to carry out a standard glucose tolerance test (75 ml of glucose per os) with the determination of glucose levels and immunoreactive insulin (IRI). Evidence in favor of insulin resistance is also a BMI over 25 and OT / OB over 0.85, as well as dyslipidemia.
Treatment At the core modern approach to the pathogenetic treatment of PCOS lies the principle of restoring impaired ovarian function
, that is, the elimination of anovulation, which in turn leads to a decrease in hyperandrogenism and the restoration of folliculogenesis. However, the study of the features of the etiopathogenesis of ovarian hyperandrogenism leads to the conclusion that the choice of methods for adequate treatment of PCOS is not an easy task.
Combined oral contraceptives - the most commonly used group of drugs for PCOS. The mechanism of action is to suppress elevated LH, normalize the LH / FSH ratio, increase the synthesis of SSSH by the liver. After cancellation, a “rebound effect” is achieved, which consists in the normalization of the hypothalamic-pituitary function, the reduction of androgen hyperproduction by ovarian tissue, the normalization of folliculogenesis and the restoration of ovulation.
Treatment is carried out according to the standard scheme: 1 tablet per day from the 5th to the 25th day of the cycle for 3-6 months. If necessary, the courses are repeated. However, it is known that long-term use of estrogen-progestin contraceptives can lead to hyperinsulinemia, thereby aggravating the main pathogenetic link of PCOS.
Some contraceptives contain progestogen components derived from 19-norsteroids (norethisterone, levonorgestrel), which have varying degrees of androgenic effects, and therefore the prescription of drugs containing these components in patients with hirsutism is limited. It is more advisable for symptoms of hyperandrogenism to use oral contraceptives with a progestogen without androgenic action.
It is possible to use progestin preparations devoid of androgenic properties in the form of monotherapy, especially with endometrial hyperplasia. Dydrogesterone is prescribed 1 tablet (10 mg) 2 times a day from 14-16 to 25 days of the cycle lasting from 3 to 6 courses.
The most effective means of stimulating ovulation in PCOS is an antiestrogen drug. clomiphene citrate . The main effects of antiestrogens are a decrease in pituitary hypersensitivity to the action of GnRH, a decrease in LH production, induction of an ovulatory LH surge, and stimulation of ovulation. The drug is prescribed at 50 mg, 100 mg per day from 5 to 9 days of the cycle until ovulation is achieved according to functional diagnostic tests, but not more than 3 courses in a row. Recently, there have been publications on the effect of clomiphene citrate on the insulin-insulin-like growth factor system. They indicated that by the 5th day of the stimulation of ovulation with clomiphene (150 mg/day), a progressive decrease (by a maximum of 30%) in the level of IGF-1 was determined. However, in a number of other similar studies, a significant decrease in basal insulin levels in response to the administration of clomiphene was not found.
The advent of drugs with antiandrogenic properties has greatly expanded the therapeutic options for PCOS. The most widely used drug is Diane-35, containing 35 mg of ethinyl estradiol and 2 mg of cyproterone acetate. In addition to the action characteristic of oral contraceptives, the drug blocks the action of androgens at the level of target cells, in particular, hair follicles. The latter leads to a decrease in hirsutism. The drug is used according to the standard scheme, as an oral contraceptive in courses of 6 or more cycles. However, it should be noted that these drugs have a negative effect on lipid and carbohydrate metabolism, which manifests itself in an increase in the level of cholesterol, low density lipoproteins, as well as an increase in hyperinsulinemia, which requires constant dynamic monitoring of these indicators in patients with PCOS. Spironolactone, which is widely used in the treatment of androgen-dependent dermopathies, also has antiandrogenic properties.
One of the main directions in modern therapy ovarian hyperandrogenism is the search and use of drugs and agents aimed at eliminating insulin resistance and compensatory hyperinsulinemia.
First of all, these are measures that ensure the reduction of excess body weight: a low-calorie diet (within 1500-2200 kcal / day) with restriction of fats and easily digestible carbohydrates, restriction of salt intake to 3-5 g per day, moderate physical activity, normalization of the work regime and rest. It is possible to use drugs that help reduce BMI, for example, orlistat, which selectively inhibits gastrointestinal lipases (“fat blocker”) or sibutramine, which blocks the reuptake of noradrenaline and serotonin in the synapses of the hypothalamic “satiation” center. Increased energy expenditure (thermogenesis) is also due to a synergistic interaction between increased norepinephrine and serotonin function in the central nervous system. This is expressed in the selective activation of the central sympathetic influence on brown adipose tissue due to indirect activation of b 3 -adrenergic receptors.
The next step is the use of drugs that improve the impaired sensitivity of tissues to the action of insulin. There are data in the literature on the reduction of hyperandrogenism and the restoration of menstrual and ovulatory function when prescribing drugs of a number of biguanides (metformin /Siofor®/, Berlin-Chemie). They potentiate the action of insulin at the receptor and post-receptor levels and significantly improve the sensitivity of tissues to this hormone. Some studies have shown a significant decrease in fasting insulin levels and 2 hours after a 75 g glucose load in women with PCOS treated with metformin. This decrease correlated with a decrease in androgen levels. It should also be noted that the use of biguanides, which normalize carbohydrate disorders, often leads to a decrease in BMI in obese patients and has a positive effect on lipid metabolism.
The world literature reports the results of the use of drugs belonging to the class of thiazolidinediones. Studies have shown that during treatment troglitazone (200-400 mg / day) improves insulin sensitivity in women with PCOS, androgen levels decrease. However, the revealed cytotoxic, hepatotoxic effect of this group of drugs limits the possibility of their widespread use. A search is underway for new drugs that selectively affect insulin sensitivity.
Despite a significant arsenal of various agents used to treat ovarian hyperandrogenism, the therapy of this pathology should be comprehensive and consistent, taking into account the leading pathogenetic link at this stage of treatment.
Treatment of women with PCOS should be aimed not only at correcting the identified symptoms of this disease, but also at preventing possible future complications. It is very important to suppress the excess secretion of androgens and induce the stability of monthly menstrual bleeding, which is successfully achieved with the use of drugs with antiandrogenic properties (Diana-35).
In case of inefficiency conservative therapy a year later, you can ask about surgical treatment - laparoscopy with wedge resection of the ovaries or their laser vaporization . Efficiency surgical treatment high (up to 90-95% ovulation recovery), and preliminary pathogenetic therapy increases the stability of the achieved result.
Literature:1. Ovsyannikova T.V., Demidova I.Yu., Glazkova O.I. Problems of reproduction, 1998; 6:5-8.
2. Ginzburg M.M., Kozupitsa G.S. Problems of endocrinology, 1997; 6:40-2.
3. Starkova N.T. Clinical endocrinology. Guidelines for Physicians, 1991; 399.
4. Givens J.R., Wiedeme E. B-endorphine and B-lipotropin levels in hirsute women: correlation with body weight. J Clin Endocr Metabol. 1980; 50:975-81.
5. Aleem F.A., McIntosh T. Elevated plasma levels of f-endorph in a group of women with polycystic ovarian desease. Fertil and Steril. 1984; 42:686-9.
6. Dedov I.I., Suntsov Yu.I., Kudryakova S.V. Problems of endocrinology. 1998; 6:45-8.
7. Francis S., Greenspan, Forshman P.H. Basic and clinical endocrinology. 1987.
8. Akmaev I.K. Problems of endocrinology. 1990; 12-8.
9. Barbieri R.L., Hornstein M.D. Hyperinsulinemia and ovarian hyperandrogenism: cause and effect. Endocrinol Metab Clin North Am. 1988; 17:685-97.
10. Barbieri R.L., Macris A., Ryan K.J. Insulin stimulates androgen accumulations in incubation of human ovarian stroma and theca. Obstet Gynecol. 1984; 64:73-80.
11. Barbieri R.L., Ryan K.J. Hyperandrogenism, insulin resistance, acanthosis nigricans: a common endocrinopathy with unique pathophysiologic features. Am J Obstet Gynecol. 1983; 147:90-103.
12. Barbieri R.L., Smith S., Ryan K.J. The role of Hyperinsulinemia in the pathogenesis of ovarian Hyperandrogenism. Fertil and Steril. 1988; 50:197-210.
13. Stuart C.A., Prince M.J., Peters E.J. Obstet Gynecol. 1987; 69:921-3.
14. Yen S.S.C. Chronic anovuletion causet by peripheral endocrine disorders. In: Yen S.S.C., Jaffe R.B. Reproductive endocrinology: physiology, pathophysiology, and clinical management. Philadelphia: Saunders W.B. 1986; 462-87.
15. Moller D.E., Flier J.S. Detection of an alteration in the insulin-reseptor gene in a patient with insulin resistance, acantosis nigricans and polycystic ovarian syndrome. N Engl J Med. 1988; 319:1526-32.
16. Burgen G.A., Givens J.R. Insulin resistance and hyperandrogenism: clinical syndromes and possible mechanisms. Hemisphera Publishing CO., Washington, DC. 1988; 293-317.
17. Speroff L., Glass R. H. Clinical gynecologic. Endocrinology and Infertility 5th ed. 1994.
18. Yoshimasa Y., Seino S., et al. Insulin resistance diabetes due to a point mutacion that privents insulin proreceptor processing./ Science. 1988; 240:784-9.
19. Dunaif A. Endocrin. Rev., 18(6): 1997; 12:774-800.
Ethinylestradiol + cyproterone acetate
Diane-35 (trade name)
(Shering AG)
Hyperandrogenism is an endocrine disease caused by increased secretion of male sex hormones in a woman's body. Androgens are produced by the ovaries and adrenal cortex. Depending on the primary cause pathology may vary. clinical symptoms.
Hyperandrogenism in women causes increased secretion of luteinizing hormone in the pituitary gland, which blocks the release of follicle-stimulating hormone and estradiol. As a result, the process of maturation of the follicle is disrupted, the release of the egg (anovulation) does not occur. High levels of androgens contribute to the formation of multiple cysts in the ovaries (polycystic ovary syndrome).
Male hormones reduce the susceptibility of peripheral tissues to insulin, which leads to an increase in blood glucose levels, impaired glucose tolerance, carbohydrate metabolism and the development of type 2 diabetes mellitus.
Classify true and idiopathic hyperandrogenism. In the first case, the level of androgens in the woman's blood is increased, and in the second, the sensitivity of peripheral tissue receptors to male hormones is increased.
Causes of pathology
What is hyperandrogenism and why does it occur? The main causes of the disease are:
- tumors, adrenal metastases;
- violation of the hypothalamic-pituitary regulation caused by injuries, tumors, inflammatory diseases of the brain;
- ovarian tumors: luteoma, thecoma;
- androgenital syndrome is a congenital pathology of the adrenal cortex, in which there is an increased production of testosterone.

In women, the causes of hyperandrogenism cause a violation of the hormonal balance, the functioning of the reproductive system, and metabolic processes in the body.
Symptoms of ovarian hyperandrogenism
The disease is of ovarian and adrenal origin - depending on the organ, which begins to intensively produce androgens. Ovarian hyperandrogenism in most cases develops against the background of polycystic ovary syndrome, less often pathology is caused by hormone-producing tumors.
PCOS is characterized by a disorder menstrual cycle, infertility, increased levels of androgens in the blood. The figure of the girl changes according to the male type, hair on the face and body begins to grow, the volume of the waist and chest increases, the fat layer is deposited in the lower abdomen. The work of the sebaceous glands is disrupted, seborrhea appears, an acne rash that cannot be treated. Stretch marks appear on the skin of the thighs and buttocks. Sleep apnea(breath holding) leads to insomnia.
Pictured is a woman with characteristic features hirsutism.

The characteristic symptoms of hyperandrogenism in PCOS are the appearance of premenstrual syndrome. Women become irritable, their mood often changes, they are worried about migraine, intense pain in the lower abdomen, swelling, soreness of the mammary glands.
The ovaries increase in size by 2-3 times, their capsule thickens. Inside the organ are found multiple cystic formations. Hormonal imbalance causes thickening and hyperplasia of the endometrium of the uterus, menstruation becomes longer, more abundant, with the release of blood clots.
Symptoms of adrenal hyperandrogenism
This type of virilization develops against the background of androgenital syndrome. This is a hereditary disease that causes increased secretion of androgens in the adrenal cortex. The congenital deficiency of organ enzymes is compensated by the body up to a certain point, but when exposed to a number of factors, hormonal balance is disturbed. Pregnancy can provoke such a condition, severe stress the onset of sexual activity.

The cause of adrenal hyperandrogenism can be hormone-producing tumors, Itsenko-Cushing's disease, hyperprolactinemia, acromegaly. Cancer cells in the reticular zone of the cortical layer produce "weak" androgens. In the process of metabolism, male hormones turn into a more active form and change the overall hormonal background of a woman. Obesity contributes to the acceleration of these processes.
Adrenal hyperandrogenism causes cyclic disturbances in the ovaries due to an increase in estrogen levels, suppression of the growth and maturation of the follicle occurs, the menstrual cycle is disturbed, and menstruation may completely stop. The process of ovulation does not occur, a woman cannot become pregnant and bear a child.

Symptoms of adrenal hyperandrogenism in girls:
- deformation of the external genital organs at birth, it is difficult to determine the sex of a child (female hermaphroditism);
- delayed sexual development, menarche begins at the age of 15–16, the menstrual cycle is irregular, accompanied by profuse blood loss;
- in girls in adolescence, signs of hirsutism are observed: hair grows on the face and body like in men;
- acne, seborrhea, skin pigmentation;
- partial atrophy of the mammary glands;
- an increase in the size of the clitoris;
- alopecia - hair loss on the head;
- the figure changes: narrow hips, broad shoulders, short stature;
- rough voice.

In women of reproductive age, adrenal hyperandrogenism leads to early abortion. This is caused by the cessation of growth of the uterus due to the formation of an inferior corpus luteum. In most girls, menstrual and reproductive function is completely disrupted, infertility develops, and sexual desire increases. Hirsutism is weakly expressed, physique does not change, metabolic processes are not disturbed.
Mixed type of hyperandrogenism
Hyperandrogenism of mixed origin is manifested by symptoms of the ovarian and adrenal forms of the disease. In women, polycystic ovaries and signs of androgenital syndrome are found.
Manifestations of a mixed type of disease:
- acne
- striae;
- elevated arterial pressure;
- violation of the menstrual cycle, amenorrhea;
- cysts in the ovaries;
- infertility, early termination of pregnancy;
- impaired glucose tolerance or high blood sugar;
- elevated levels of low density lipoproteins.

Hyperandrogenism can be caused by systemic diseases that affect the adrenal cortex, ovaries or brain, and disrupt metabolism. These are pituitary adenomas, anorexia nervosa, schizophrenia, diabetes Type 2, acromegaly, prolactinoma.
Peripheral and central hyperandrogenism
With damage to the central nervous system, inflammatory, infectious diseases or intoxication of the body, the secretion of gonadotropic hormones of the pituitary gland, which are responsible for the production of luteinizing and follicle-stimulating hormone, can be suppressed. As a result, the process of maturation of the follicle in the ovary and the synthesis of sex hormones are disrupted, androgen production increases.
Women show symptoms of polycystic disease, ovarian dysfunction, menstrual disorders, skin rashes, PMS.

Peripheral hyperandrogenism is caused by an increase in the activity of a skin enzyme, sebaceous gland 5-α-reductase, which converts testosterone to the more potent androgen dihydrotestosterone. This leads to hirsutism of varying severity, the appearance of acne vulgaris.
Hyperandrogenism during pregnancy
In pregnant women, an increase in androgen levels is the cause of spontaneous abortion. The most dangerous terms are the first 7–8 and 28–30 weeks. In 40% of patients, intrauterine fetal hypoxia is observed, most often this occurs in the third trimester. Another complication is late toxicosis, while kidney function worsens, blood pressure rises, and body edema appears.
Hyperandrogenism during pregnancy can lead to premature discharge of amniotic fluid, complicated childbirth. Changes in the hormonal background negatively affect the development of the child, in infants cerebral circulation may be disturbed, there are signs of intrauterine malnutrition.

Hyperandrogenism and pregnancy are reasons for urgent hormone therapy to prevent abortion and other complications. Women who have previously had miscarriages, miscarriage, increased levels of male hormones should be carefully examined at the stage of pregnancy planning.
Diagnosis of the disease
Diagnosis - hyperandrogenism is established according to the results of laboratory tests on the level of hormones. With polycystic ovary syndrome in the blood of a woman, the level of testosterone, androstenedione, luteinizing hormone increases. The concentration of FSH, prolactin, DHEA in the blood and 17-KS in the urine remains within the normal range. The ratio of LH/FSH is increased by 3-4 times. With hormone-dependent ovarian tumors, the level of testosterone and prolactin in the blood is significantly increased.

The mixed form of the disease is characterized by a slight increase in the level of testosterone, LH, DHEA-S in the blood and 17-KS in the urine. The concentration of prolactin is normal, and estradiol and FSH are reduced. The ratio of LH / FSH is 3.2.
To determine the primary cause of hyperandrogenism, tests are carried out with Dexamethasone and chorionic gonadotropin. A positive hCG test confirms polycystic ovarian disease, which causes a hormonal imbalance. A negative response indicates the adrenal nature of hyperandrogenism.

The Abraham test allows you to identify a disease of adrenal origin, with the introduction of synthetic glucocorticoids, the synthesis of ACTH in the anterior pituitary gland is suppressed, which stops the stimulation of the adrenal cortex. If the result is positive, it is adrenal hyperandrogenism, a negative response may be a sign of a cortical tumor.
Additionally, ultrasound of the ovaries is performed to detect cysts, changes in the size and structure of the organ. Electroencephalography, MRI, CT of the brain are indicated for suspected damage to the pituitary gland.
Treatment Methods
Therapy is prescribed individually for each patient. Androgen receptor blockers reduce the effect of male hormones on the skin, ovaries (Flutamide, Spironolactone). Androgen secretion inhibitors inhibit the production of testosterone by the endocrine glands (cyproterone acetate). These funds restore the balance of hormones, eliminate the symptoms of pathology.
Hyperandrogenism of the adrenal glands is compensated by glucocorticoids, which suppress the excess of androgens. Women are prescribed Dexamethasone, Prednisolone, they are also taken during pregnancy if the expectant mother has an increased level of testosterone. It is especially important to be treated in a timely manner for girls who have close relatives with congenital androgenital syndrome. The dosage and duration of the medication is prescribed by the doctor.

Hormonal treatment of hyperandrogenism is carried out with glucocorticosteroids combined oral contraceptives(Diana-35), GnRH agonists. Mild hyperandrogenism is treated with such drugs. ovarian origin, PCOS.
Non-drug treatment
To restore hormonal balance, women are advised to regularly engage in moderate physical activity to give up bad habits healthy lifestyle life. It is important to adhere to the diet, to make a balanced diet that excludes coffee, alcohol, carbohydrates, animal fats. It is good to eat fresh fruits, vegetables, dairy products, dietary meats and fish. Pharmaceutical preparations are taken to compensate for the deficiency of vitamins.

Treatment folk remedies can only be carried out in combination with the main therapy. You should first consult a doctor.
Hyperandrogenism causes disturbances in the work of many organs and systems, leads to the development of adrenal and ovarian insufficiency, infertility, and type 2 diabetes mellitus. To prevent the appearance of symptoms of hirsutism, skin rashes, metabolic syndrome, hormone therapy is indicated.
Bibliography
- Kozlova V.I., Pukhner A.F. Viral, chlamydial and mycoplasmal diseases of the genitals. Guide for doctors. St. Petersburg 2000.-574 p.
- Miscarriage, infection, innate immunity; Makarov O.V., Bakhareva I.V. (Gankovskaya L.V., Gankovskaya O.A., Kovalchuk L.V.) - "GEOTAR - Media". - Moscow. - 73 p.-2007.
- Emergency conditions in obstetrics and gynecology: diagnosis and treatment. Pearlman M., Tintinalli J. 2008 Publisher: Binom. Knowledge Lab.
- Adamyan L.V. etc. Malformations of the uterus and vagina. – M.: Medicine, 1998.
Hyperandrogenism of mixed genesis can be manifested by signs of the ovarian and adrenal forms of the disease. For any woman of reproductive age, one of the most unpleasant diseases is. This disease is found in 4-6% of women of reproductive age. Among the main causes of this problem should be noted an excess of male hormones and strong physical exertion.
Symptoms of ovarian hyperandrogenism
The main symptoms of this disease include cosmetic manifestations - acne, hair loss and hirsutism. In addition, hyperandrogenism can also have gynecological manifestations. Very often, women suffering from this disease have menstrual irregularities and polycystic ovary syndrome. Another unpleasant gynecological symptom is infertility. It is also worth noting that obesity, diabetes mellitus, dyslipidemia and the manifestation of signs of male hormones can be a symptom of this disease. These signs include:
- facial hair growth;
- change in voice timbre;
- shoulder extension.
Diagnostics
Even mild hyperandrogenism of ovarian origin can be diagnosed. Therefore, women who are faced with the above symptoms should definitely contact specialized specialists. Diagnosis of this disease is usually based on the results of ultrasound, as well as hormonal and clinical examination data. Patients suffering from hyperandrogenism of ovarian origin, as a rule, have an increased body mass index and a hirsute number of 15.2 ± 0.6. Also, during the diagnostic examination, the presence of high concentration testosterone, luteinizing and follicle-stimulating hormones. Ultrasound may show an increase in ovarian volume and stromal hyperplasia.
Treatment of pathology
Treatment of hyperandrogenism of ovarian origin should be prescribed exclusively by a specialized specialist. The method of treatment directly depends on the goals of the patient. If she plans to conceive children in the future, then clomiphene is most often used for treatment. In the event that the patient is not going to restore fertility, then the attending physician will most likely prescribe treatment with oral contraceptives. Due to this, the patient's testosterone and androstenedione levels will significantly decrease. An alternative to this treatment is spironolactone. Also, for the treatment of this pathology, a surgical method can be used - surgical removal of the ovaries.
Mild hyperandrogenism of ovarian origin, PCOS are amenable to hormonal treatment with glucocorticosteroids, combined oral contraceptives, GnRH agonists.
What is adrenal hyperandrogenism
Virilization of adrenal genesis is manifested by the development of adrenogenital syndrome (AGS) - as a result of congenital dysfunction of the adrenal cortex. This is a genetically determined disease inherited in an autosomal recessive manner. AGS is explained by the inferiority of enzyme systems in the gland: in 80-90% of patients, inferiority is manifested by a deficiency of C21-hydroxylase.
Pathogenesis (what happens?) during adrenal hyperandrogenism
The main pathogenetic mechanism of AGS is a congenital deficiency of the C21-hydroxylase enzyme involved in the synthesis of androgens in the adrenal cortex. The formation of C21-hydroxylase is provided by a gene localized in the short arm of one of the 6th pair of chromosomes. Pathology may not manifest itself in the inheritance of one pathological gene and manifests itself in the presence of defective genes in both autosomes of the 6th pair. Partial blockade of steroid synthesis in congenital C21-hydroxylase deficiency increases androgen synthesis.
Until a certain age, a mild deficiency of C21-hydroxylase in the adrenal glands is compensated. With an increase in the function of the adrenal glands (emotional stress, the onset of sexual activity, pregnancy), the synthesis of steroids is disrupted towards hyperandrogenism, which in turn inhibits the release of gonadotropins and disrupts cyclic changes in the ovaries.
In this case, in the ovaries are possible:
- suppression of growth and maturation of follicles on early stages folliculogenesis leading to amenorrhea;
- inhibition of growth, maturation of the follicle and the egg, which is not able to ovulate, which is manifested by anovulation and oligomenorrhea;
- ovulation with a defective corpus luteum: despite regular menstruation, there is an insufficiency of the luteal phase of the cycle.
With all these variants of ovarian hypofunction, infertility occurs. The frequency of miscarriage in AGS reaches 26%.
Symptoms of adrenal hyperandrogenism
Depending on the severity of C21-hydroxylase deficiency and, accordingly, hyperandrogenism, the classical form of AGS and mild, or late (pubertal and post-pubertal) forms of AGS are distinguished.
The classical form of AGS is accompanied by congenital adrenal hyperplasia. Such virilization leads to pathology of the external genitalia (false female hermaphroditism) and incorrect sex determination at birth. Children with such a pathology undergo surgical correction of the iol, further treatment and observation is provided by a pediatric endocrinologist.
In puberty, the main complaints of patients are hirsutism, acne, irregular menstruation. Menarche occurs at the age of 15-16, in the future menstruation is irregular, there is a tendency to oligomenorrhea. In this period, hirsutism is more pronounced: hair growth along the white line of the abdomen, on the upper lip, inner thighs. The skin is oily, porous, with multiple acne, extensive hyperpigmentation spots. In the physique, the influence of androgens is also noticeable: unsharply pronounced masculine features with broad shoulders and a narrow pelvis, shortening of the limbs, short stature. After the appearance of hirsutism, the mammary glands are hypoplastic.
In patients with post-pubertal form of AGS, menstrual and reproductive functions are impaired. The post-pubertal form of AGS manifests itself as an early termination of pregnancy. The severity of reproductive dysfunction depends on the degree of C21-hydroxylase deficiency: from a decrease in reproductive function due to anovulatory cycles to miscarriage due to the formation of an inferior corpus luteum. Hirsutism is slightly expressed: poor hair growth of the white line of the abdomen, individual hairs above the upper lip. The physique of the female type, the mammary glands are quite developed, metabolic disorders are atypical.
Diagnosis of adrenal hyperandrogenism
When questioned, menstrual irregularities are revealed in the sisters and relatives of the patient on the maternal and paternal lines. With AGS, excessive sexual hair growth develops early and quickly, menarche occurs late, and further menstruation is irregular.
For AGS pathognomonic athletic physique, hypertrichosis, acne, moderate hypoplasia of the mammary glands. AGS is not accompanied by an increase in body weight, unlike other endocrine disorders with hypertrichosis.
The main role in the diagnosis of AGS belongs to hormonal studies. To clarify the origin of androgens, hormonal studies are carried out before and after the dexamethasone test. A decrease in the levels of 17-KS in the urine, testosterone and dehydroepiandrosterone in the blood after taking drugs that inhibit the release of ACTH indicates the adrenal origin of androgens.
Diagnosis of postpubertal AGS is based on:
- hypertrichosis and a decrease in reproductive function in the patient's sisters on the maternal and paternal lines;
- late menarche (14-16 years);
- hypertrichosis and irregular menstruation since menarche;
- the viril features of the morphotype;
- an increase in the content of 17-KS in the urine, testosterone and dehydroepiandrosterone in the blood and a decrease in their level to normal after taking dexamethasone.
Ovarian ultrasound data indicate anovulation: follicles of different maturity do not reach preovulatory sizes. Basal temperature with a stretched first and shortened second phase indicates insufficiency of the corpus luteum, the type of vaginal smears is androgenic.
Treatment of adrenal hyperandrogenism
Choice medical preparations in patients with AGS, it is determined by the goal of therapy: normalization of the menstrual cycle, stimulation of ovulation, suppression of hypertrichosis.
In order to correct violations of the hormonal function of the adrenal cortex, glucocorticosteroid drugs are used. The dose of dexamethasone is determined in accordance with the level of 17-KS in the urine, dehydroepiandrosterone and testosterone in the blood (during treatment, the level of these hormones should not exceed the upper limit of normal). The effectiveness of treatment, in addition to hormonal studies, is controlled by measuring basal temperature and monitoring the menstrual cycle. With an inferior second phase of the menstrual cycle, it is necessary to stimulate ovulation from the 5th to the 9th day, against which pregnancy often occurs. In order to avoid spontaneous miscarriage, glucocorticosteroid therapy should be continued, its duration is determined individually.
If a woman is not interested in pregnancy, and the main complaint is hypertrichosis and pustular skin rashes, preparations containing estrogens and antiandrogens are recommended. Diane effectively affects hypertrichosis, especially in combination with androcur in the first 10-12 days. Diana is prescribed from the 5th to the 25th day of the menstrual cycle for 4-6 months.
Veroshpiron gives an antiandrogenic effect, as a result of suppression of the formation of dihydrotestosterone from testosterone in the skin, hair follicles and sebaceous glands. Veroshpiron is prescribed 25 mg 2 times a day. The use of veroshpiron for 4-6 months reduces the level of testosterone by 80%, but there was no decrease in the level of corticotropic and gonadotropic hormones.
The use of synthetic progestins also reduces hypertrichosis, but the use of these drugs in women with AGS should not be long-term, since suppression of gonadotropins is undesirable in depressed ovarian function.
Which Doctors Should You See If You Have Adrenal Hyperandrogenism?
Gynecologist
Promotions and special offers
medical news
On October 12, 13 and 14, Russia is hosting a large-scale social campaign for a free blood coagulation test - “INR Day”. The action is timed to world day the fight against thrombosis.
07.05.2019
The incidence of meningococcal infection in the Russian Federation in 2018 (compared to 2017) increased by 10% (1). One of the most common ways to prevent infectious diseases is vaccination. Modern conjugate vaccines are aimed at preventing the occurrence of meningococcal infection and meningococcal meningitis in children (even the most early age), teenagers and adults.
25.04.2019
A long weekend is coming, and many Russians will go on vacation outside the city. It will not be superfluous to know how to protect yourself from tick bites. Temperature regime in May it promotes the activation of dangerous insects...
Viruses not only hover in the air, but can also get on handrails, seats and other surfaces, while maintaining their activity. Therefore, when traveling or in public places, it is advisable not only to exclude communication with other people, but also to avoid ...
Return good vision and say goodbye to glasses forever and contact lenses is the dream of many people. Now it can be made a reality quickly and safely. New opportunities laser correction vision is opened by a completely non-contact Femto-LASIK technique.



















