Mixed acid is among carbohydrates. In this case, the bactericidal properties of lactic acid are used. Milk Acid in the vagina
I often ask my students - future sports doctors -vopros about the fact that they think about lactic acid. And their usual answer: "Nothing good!" They blame her in everything - from pain and cramps in muscles to fatigue and injuries. It is considered as a by-product, which should be avoided at any cost.
Do you know, "I say to them, - that lactic acid plays a major role in the process of energy generation during training? And it is not at all a harmful by-product of metabolism. It gives energy, contributes to the absorption of carbohydrates and serves as fuel for the liver in the production of glucose and glycogen. In fact, lactic acid is a natural tool designed to help our body to cope with stressful situations. "But there is a reverse side of the medal. When the body produces milk acid, it breaks it into lactate ion (lactate) and hydrogen ion. In lactic acid. And is actually an acid. It interferes with the electrolytic signals of nerves and muscles, slows down the energy reactions and weakens muscle contractions. It is them caused by the burning that you feel with intense training. So, if you feel fatigue - blame nothing else like a hydrogen ion.
Kumiz is a very popular drink among Kyrgyz, Tatar and Kalmuki, nomadic tribes in Eastern Russia and Asia. On the contrary, Kefir is a national drink of climbers in the Caucasus and Ossetia. It used to be believed that kefir is easier to digest than milk, which indicates that part of the casein was dissolved during his fermentation. In other words, Kefir was considered semi-defined milk. Currently, following the studies of Hayema \u200b\u200band Rovigi, the foregoing can no longer be considered true, since they convincingly demonstrated that any positive effects observed with kefir should only be associated with a low percentage of lactic acid contained in it.
But, as a rule, Laktat accuses "for the company". Although, in reality, our body relates to him perfectly. It is extremely fast fuel for the heart and muscles. Laktat plays a vital role in ensuring the body's stable supply of carbohydrates, even during physical exertion lasting for many hours.
Kefir is useful in some cases, but cannot be recommended as food for long-term use, when it is necessary in order to cope with the consequences of decay processes in the intestine. Kefir is formed as a result of simultaneous alcoholism and dairy fermentation. In fact, it contains one percent of pure alcohol, but the daily consumption of even this small amount of alcohol is undesirable. Another objection to the use of kefir is its volatile microflora, which is not yet sufficiently studied.
Laktat is a friend of all those who train with burdens, a friend of football players, trialors, runners for long distances, swimmers and cyclists. When you learn more about this substance, everything will appear completely in a different light. Understanding the action of lactic acid, you can increase your energy level and defeat fatigue!
From dairy fermentation, and not alcohol makes kefir useful, this is the only reasonable decision that it will be replaced by yogurt, which does not contain any traces of alcohol, made of a clean culture of microorganisms producing milk acid, which undoubtedly surpasses all other yogurt. The fact that so many people of all ages consume a large number of yogurt and brings much benefit from its use, is a guarantee and confirmation of its value. A well-known African Nailire researcher in a letter to us expresses his surprise, watching the preserved appearance and the lack of unstasive changes among the local population in the Mossamed, which he did not attend many years.
Milk Acid is truly "royal" metabolit
Milk acid is formed during glucose decay. Sometimes called "blood sugar", glucose is the main source of carbohydrates in our organism. This is the main fuel for the brain and the nervous system, as well as for the muscles during physical exertion. When glucose is cleaving, cells produce ATP (adenosine trifhosphate), which provides energy most chemical reactions in the body. The ATP level determines how quickly and how long our muscles will be able to decline when exercise.
Dr. Lima confirms that a large number of people known for their extraordinary durability can be found among the population in the region to the south of Angola, and although they are weak and dry, the elderly function well and are capable of long pedestrian crossings.
Stamen Grigorov, a student from Bulgaria, reports the surprisingly large number of apartment buildings on the territory of Bulgaria, where the yogurt is one of the main elements of the diet of these people. Over the past 10 years of his life, she consumed only rye bread, yogurt and cheese. An employee named Ambroz Jeanne, who lived in Verden, reached a 111 year old age, eats with barley breads and yogurt. Woman named Nicole Mark, who died aged 110 years in the castle of Collenberg. This woman had a hump and paralysis lower extremities.
The production of lactic acid does not require the presence of oxygen, so this process is often called "anaerobic metabolism". Many believe that the muscles produce milk acid, when oxygen is inconed. In other words, you are in an anaerobic state. However, scientists argue that lactic acid is formed in the muscles receiving enough oxygen. An increase in the amount of lactic acid in the bloodstream indicates only that its level of receipt exceeds the removal level. Oxygen does not play a significant role here.
The diet basically consisted of yogurt and black bread. This woman is still alive and performs daily homework, sewing, and although it goes bent by the time she has a steady gait. The daily diet of this old woman consists of barley bread and yogurt. The American Lady named Jenny Raid wrote me that her 84-year-old father must be his longevity and good health yogurt, which he drank for over 40 years.
When using yogurt, his taste is indicative, and the closer to the natural fermented yogurt, the better. Let me, however, one is completely clear, because of its everyday use, we should not lose sight of the fears of natural yogurt production. Raw milk contains a variety of microbial flora, including microbes that can cause serious visceral disorders. Also in milk the bacillus of cattle tuberculosis is common. According to studies conducted by Haim, the most common bacillins flourish in milk even after he became sour.
Lactate dependent on the production of ATP is very slightly, but has a greater speed. This circumstance makes it ideal to use it as a fuel when the load exceeds 50% of the maximum. When rest and submaximal load, the body prefers split fats for energy. With loads of 50% of the maximum (intensity threshold for most training programs), the body is rebuilt on the predominant carbohydrate consumption. The more carbohydrates you use as fuel, the greater the production of lactic acid.
In such conditions, Bacillus Tifa was found alive on the 35th day and died only after a 45-day stay in a well fermented yogurt. The taste, as fresh milk, almost always contains traces of the fecal mass of the cow, other harmful microbes penetrate it and remain alive, despite the acid coagulation of milk. It is true that lactic acid bacteria prevent the spread of these harmful microbes, which cause the disintegration of organisms, but cannot destroy them.
In addition, raw milk often contains various forms and mushrooms that contribute to the development of such harmful microbes like cholera bacillus and stomach. Therefore, the further use of milk, which wanders, naturally increases the risk of entering harmful organisms in the human body. For the production of yogurt, which does not contain harmful bacteria, it is necessary to first sterilize raw milk, and then add useful bacteria. But this does not include yeast at all, as many thinks, but, on the contrary, other enzymes cultivated by the appropriate scientific way are added.
Metabolic mediator
The body uses milk acid as a biochemical mediator with carbohydrate exchange. Carbohydrates are absorbed and circulated from the intestines into the liver mainly in the form of glucose. However, instead of entering the liver for the subsequent transformation into glycogen, most of the glucose from food carbohydrates, bypassing the liver, comes directly into the bloodstream, reaches the muscles and turns into a lactic acid. She, in turn, comes back into the blood, then into the liver, where it is used to create glycogen. Your body forms most of its hepatic glycogen not directly from blood glucose, but through the formation of lactic acid. The process of this scientists is called a "glucose paradox".
Many fabrics, especially skeletal muscles, are constantly synthesized and used milk acid. Its level in the blood reflects the balance between production and consumption.
Rist and Hoar discover that the Egyptian Leben contains a microbial flora of at least five different species - Three bacterial and two types of yeast. The first causes acid fermentation, and the second alcoholic fermentation. An analogy between the Egyptian "Lebed" and Kefira is quite accurate, because in both cases it is about milk and alcohol fermentation. The clarifications that we have already done when we talked about kefir, also belong to the Egyptian "Lebena".
With the helpful assistance of the professor of massol from Geneva, we were able to deliver a sample of Bulgarian yogurt, whose microflora was especially well studied by His student Grigorov. In our own laboratory, Mikhelson began the study of Bulgarian milk. Both researchers discovered various microbes in it, including lactic acid bacteria and different types yeast. In general, Flora Bulgarian yogurt is similar to the flora of the Egyptian "Lebed", and therefore their effect is identical. Studies conducted in BCM conducted in our laboratory at the Pasteur Institute showed wide spectrum Microorganisms, including the real pink yeast fungus, which contributes to a large extent, the growth and viability of bacteria of abdominal and cholera, demonstrated by our experiments on rabbits.
The production of lactic acid is proportional to the amount of carbohydrates split for energy needs in the tissues. When using carbohydrates, they are quite large part turns into lactate, which is then used with the same tissues as fuel or is transferred by blood flow to other fabrics for the energy target. The rapid use of carbohydrates as fuel, such as, for example, during intensive physical activity, accelerates the production of lactic acid. Temporarily, it begins to accumulate in muscles and blood, because it cannot be used as a fuel very quickly. If you slow down the pace of exercise or generally stop the occupation, the level of use of lactate is soon levels with the level of its production. Dr. George Brooks (George Brooks), Professor of the Faculty of General Biology of the University of California, described the dynamics of production and use of lactic acid in the metabolic process in its so-called "lactate's shuttle theory" ("Lactate Shuttle Theory"). It shows the leading role of lactic acid in carbohydrate metabolism and the importance of it as fuel for metabolism. In an exclusive interview, Dr. Brooks said: "To lactic acid, in general, it is bad. But if the athletes were able to learn how to control this chemical process and use it, we could train tougher and longer. Regulation of lactic acid level is the key to Success in high-intensity sports! "
Therefore, if milk is not sterilized properly, and then populated by any of the above crops, the products of this process are a threat to health and life. The only safe technology is to isolate and store the clean culture of certain lactic acid microbes and their placement in sterilized milk, resulting in fermented milk without even the smallest amount of alcohol.
With this microbe, unlike pure culture from it, we have prepared our yogurt, excluding any other microbes and enzymes. Within a long period of use of this yogurt, it was found that fat contained in milk reduces its wellness effect. It is best to remove cream or add other lactic acid bacteria to emulsify this substance. After the preparation and cooling of fresh milk, it was planted with a clean culture of lactic acid bacilli.
Heart, slow-growing muscle fibers and breathing muscles prefer to use lactate as a fuel during exercise. In the heart, for example, its consumption increases significantly with increasing load, and the use of glucose remains unchanged.
The incubation period varies depending on the temperature at which the bacteria treated with milk are stored. The result in the implementation of these recommendations is a cream yoghurt of a pleasant taste, which has the ability to interrupt the process of rotting, developing in the intestinal tract.
This milk should be consumed daily in an amount from one to one pint and a half during or between or between meals. If you follow these recommendations, the intestine function is regulated and the kidney secretion increases. The use of fermented milk, as indicated above, can be recommended for all diseases of the digestive tract and urinary authorities, as well as for many skin disease.
Milk Acid is a very "fast" fuel, which can help athletes in improving performance. After taking high-car food, the concentration of blood both glucose and lactic acid increases. But the lactate level rises slightly, as it is removed quickly enough. The body turns glucose (which moves in the blood not so fast) into lactate, so it reaches the goal faster. The use of lactic acid as a "intermediary" helps to get rid of carbohydrates obtained with food without lifting insulin level and stimulation of fats synthesis. During training, this lift is not needed, as it lowers the availability of carbohydrates, extremely necessary for intensive metabolism.
The microbe in which we experiment, has the ability to exist and spread at sufficiently high temperatures. With his population in the human intestine, he quickly adapts to life in the environment and becomes an important part of the intestinal flora, as evidenced by Coente. Yoghurt, made in accordance with the instructions shown above, with a clean culture of a particular microbe, which we described, was analyzed by Fuard at the Pasteur Institute. At the time when milk was ready to use, the popard found that it contained 10 cubic centimeters of lactic acid per liter.
Why is lactic acid so important in the adjustment of metabolism? There is no accurate answer yet, but there are certain physiological reasons. Milk acid, in contrast to glucose and other types of fuel, has a smaller size of molecules, so it is easier to pass from one tissue to another. It penetrates through the cell membranes by means of an instant process called "Light Transport" (Facilitated Transport). For other types of fuels, slower transport systems are required - such as insulin. Thus, lactate gets faster and in large quantities in cells and blood flow. Muscular cells with large glycogen reserves cannot release significant amounts of such a potential energy source as glucose, because they do not have a key enzyme responsible for the production of free glucose to release it into the blood.
In addition, there was a high percentage of casein, which became soluble substance. The latter observation shows that the protein contained in milk has been turned into a disappointment, unlike any other milk. As for the mineral content of this milk, the researcher found that 68% of calcium phosphate turned into a soluble substance.
All the facts still testify to the excellent qualities of this milk. The reader may be surprised to see that it is useful to take such a large number of microbes and especially against the background of the general conviction that all microbes are harmful and even dangerous to health and life. This is a big mistake, because we contain many microbes that are necessary for our health. One of them is the lactic acid bacteria among them. He used pure cultures of this bacterium in the treatment of diarrhea in infants, and Tissy in practice applied these cultures with any problems with the digestive tract in children, as well as in adults.
Milk Acid and fatigue
"Work to burning!" - Says your aerobics instructor. Full famous factthat with intense physical exertion of lactic acid causes burning, associated with muscular fatigue. Probably so. Hydrogen ions interfere with muscle contraction and energy producing reactions.
For many years we have been preparing the milk described above, first sterilizing it, and then inhabit it with a clean culture from our microbe. We accept a lot of this yogurt every day and very satisfied with the results. After this long experiment, we consider it necessary to express our positive opinion on this issue.
Due to the foregoing, we naturally came to the opinion that in the fight against the intestinal breakdown, bacteria producing lactic acid have an undeniable benefit. Finally, to confirm that with the help of this yogurt, obtained in this way, we have a medicine from old age or means to continue human life, let the time personal experience And the observations made give an answer to this question.
During training nervous system Protects the heart, brain and muscles from oxygen deficiency. The level of lactic acid in the muscles is an important signal for it when the blood distribution by body. When the system determines that oxygen supply can be reduced somewhere, it reduces blood flow there than causeing fatigue.
However, lactic acid is responsible not for all types of fatigue during training. With loads requiring great stamina, such as marathon running or triathlon, its level in the blood does not change, despite the fact that production increases. This is because the possibilities of the body according to its production correspond to its ability to use it as fuel. At the beginning of the race there is a significant increase in the level of consumption of glucose muscles and glycogen cleavage. This is an increased temp carbohydrate exchange It causes an increase in the production of lactic acid and an increase in its blood content.
As soon as blood is sent to working muscles, you can "forward" lactate to other fabrics for energy generation. At the same time, its level in muscles and blood decreases, although the body continues to produce it in large quantities. Often in the process of race or training you feel a sudden relief. This feeling is called "second breathing". Studies have shown that during exercise, the level of production and removal of lactic acid is 300-600% more than at rest, even if the oxygen consumption has stabilized at the submaximal level.
Muscle pain and convulsions
Milk Acid is not the cause of pain and cramps in the muscles. The pain appearing in the muscles the next day after training, caused by damage to muscle fibers and their inflammation. Cramps are caused by muscle receptors, which are overexcited by fatigue muscles. Many athletes use massage, hot tubs and other methods of relaxation to remove lactic acid from muscle fibers in order to get rid of pain and cramp. Although such methods have their useful parties, getting rid of lactic acid is not one of them. Laktat is used by muscles as fuel as during training, so when recovery, and it does not remain in them like recycled engine oil.
Cut the milk acid to work for you
Properly compiled training program, combining periods of high-intensity workouts with stamina training, can accelerate the removal of lactic acid. Fortunately, most of the training programs are built precisely. Your body must learn to quickly remove lactat for follow-up successful speeches at competitions.
The level of lactic acid exchange helps you run, swim or ride a bike faster. To increase the ability of the body to use lactate as a fuel, it is necessary to increase the level of its maintenance in the muscles during training. Training S. large content The lactate in your system stimulate the body to produce enzymes, accelerating its use. A number of studies have proven the importance of lactate content in sports drinks. Athletes learn to tolerate this so-called "burning". Scientists call it "addiction." WinCe Lombardi (Vince Lombardi), the immortal coach of the team "Greenbay Packers", once said: "When the move causes pain, pain causes the movement." If he was a professor of physiology, then his statement would have sounded like this: "When the level of lactate in the muscles increases, the pain becomes a habit." It is good that he was a football coach.
With a highly intense interval training, the cardiovascular system is adapted, enhancing the supply of oxygen into muscle and other fabrics. Therefore, you will have to split a smaller amount of carbohydrates to produce lactic acid. In addition, the best blood circulation helps speed up its delivery in fabric and removal from blood flow.
Training on endurance cause muscle adaptation, which also accelerates the removal of lactic acid. Classes running, swimming or cycling cause the greatest development of microcirculation and functional power of mitochondrial cells of skeletal muscles. With an increase in this ability, the use of fatty acids as a source of energy increases and, thus, lactate formation is reduced. With an increase in the functional ability of muscular mitochondria, the removal of lactic acid from the body is also faster.
Meals also plays an important role. Intense and hard training depletes glycogen stocks in muscles and liver. Therefore, all athletes working for endurance are needed rich in carbohydrate diet.
Carbohydrates ensure the speedy production of glucose, so the athlete feels great and has a source of rapid energy. Moreover, glucose contributes to the reinforcement of glycogen reserves during the rehabilitation period. When the level of glucose in the blood and glycogen in the muscles is restored, glucose becomes a source of lactate formation that helps to fill the glycogen reserves in the liver.
Milk acid CH3Cnonson is formed as a result of anaerobic conversion of carbohydrates with milk acid bacteria. Milk acid is an organic monoxide oxic acid. The hydroxyl group of this acid can be in two (A and B) the positions of the carbon chain. Therefore, two types of lactic acid are distinguished: A-oxypropional CH3CNOson and B-oxypropional CH2Oxn2Cone. An industrial significance is a-oxypropionic acid produced in the process of lactic acid fermentation.
Milk Acid is widely used in the chemical, food, pharmaceutical industry. In the USSR, in industrial environments, foodty milk is obtained by deep cultivation using Bacterium Delbruckii bacteria (synonym Lactobacillus Delbruckii), which belong to homoformal thermophilic bacteria with a development optimum of 48-50 ° C. The production value of these microorganisms is that the temperature maximum for their development is in the range of 54-56 ° C, and intensive acid formation is provided at a relatively high temperature - 50 ° C. Such a temperature creates elective conditions. Most microorganisms do not develop at the same time, since the specified temperatures are far beyond the limits of optimum and maximum for their development. The production of lactic acid includes the following main technological steps: laminating fermentation, Processing of a fermented solution and filtration, calcium lactate cleavage, evaporation of lactic acid.
The formation of lactic acid from glucose when fermented by homofermentative lactic acid bacteria occurs according to the equation

The total equation for the conversion of glucose into lactic acid using an enzyme system of lactic acid bacteria can be represented in this form:
Glucose splitting occurs according to the PDF path, the bacteria has all the necessary enzymes for this, including aldolaz. Hydrogen, stepping during the dehydrogenation of trioseophosphate, is transmitted to the pyruvate. The diagram of lactic acid biosynthesis is presented below.
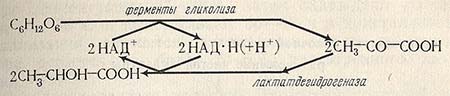
Another variant of the scheme of lactic fermentation includes the decay of glucose to pyerogradic acid and the restoration of peeling acid to dairy
Milk Acid is an unstable chemical compound, and, depending on the conditions of production and storage, it easily forms dehydration products, called lactic acid anhydrides.
Milk acid crystals at atmospheric pressure quickly melted with the formation of a colorless syrupy fluid of the specific gravity of 1.21 odorless with sharply sour taste.
Laminating fermentation
For the production of lactic acid use a wide variety of carbohydrates. In industry, acid is usually obtained from such species of raw materials, which contain glucose, sucrose and maltose. This raw material can serve as refinery, molasses, starch (corn, potato), pre-operated malt. The concentration of sugar in the fermented medium, depending on the type of raw materials and fermentation conditions, can range from 5 to 18%. For fermentation of sulfite slots, you can use lactic acid bacteria like L. Plantarum. They ferment the hydrolyzates containing pentoses (xylose, arabinose) with about the same yield of acetic and lactic acids. The separation of these formed acids is carried out by the method of distillation from fermented solutions.
In industrial conditions, lactic acid is obtained in deep way with the help of the culture of L. Delbruckii. Melassion, sucrose, starch hydrolyzates are used as the main raw material. The concentration of sugar in the medium is 5-20%, pH 6.3-6.5. During the fluid fermentation, the mediums are maintained using chalk, which is added 3-4 times a day. Local acid fermentation is carried out with a strictly constant temperature of 50 ° C. The decrease in temperature to 46-48 ° C causes a sharp weakening of the biochemical activity of culture and contributes to the development of foreign microflora. The increase in temperature, for example, up to 53-55 ° C, also causes the inactivation of the culture and the slowing down of fermentation.

A positive effect on lactic acid fermentation is provided by biologically active substances. For this purpose, a malt sprout stretch is added to the nutrient medium. With normal fermentation, 1-1.5% sugar is ferment in bacteria per day, and the entire fermentation cycle ends in 7-11 days. At the same time, the amount of unkind sugar is 0.5-0.7%, and the concentration of calcium lactate is 10-15%.
Ranted solution and filtration
For the separation of chalk and colloids, the raven solution is heated to 80-90 ° C, and then treated with a hazed lime to a weakly alkaline reaction and defended for 3-5 hours. To remove the coarse suspension and solid particles, the saturated layer of calcium lactate solution is decanted. The solution pump pumps to the filter press. The filtering is carried out at a temperature of a calcium lactate solution 70-80 ° C through a pre-heated filter press. The resulting filtrate is evaporated to a concentration of 27-30%, then cooled to a temperature of 25-30 ° C and withstand 36-48 hours in a crystallizer. Crystallization is considered complete if in the uterine: the solution remains no more than 5-6% dissolved calcium lactate.
Calcium lactate splitting
Washed cold water Calcium lactate is separated on the centrifuge and melted. In order to prevent lactate from charring, the splitting of calcium lactate with sulfuric acid with the release of free lactic acid is carried out at 60-70 ° C. This reaction passes in accordance with the equation
For the separation of iron ions, the resulting raw milk acid at a temperature of 65 ° C is treated with yellow blood salts. The sediment falls the Berlin Azure. Heavy metals and arsenic are precipitated sodium sulfate and sulfur barium. In order to release lactic acid from coloring substances, active coal is used. After treatment, the resulting mixture is filtered, and the gypsum precipitate is washed to extract the remaining lactic acid.
Eating lactic acid
After cleavage of crystalline calcium lactate and subsequent treatment, a 18-20% concentration is obtained, which is evaporated to achieve a 40% concentration. Equipment of acid is produced in vacuum devices at a residual pressure of 10-15 kPa and a pressure pressure of 0.2 MPa. Extracted up to 40% milk acid brighten with active coal and treated with yellow blood salts. After brightening the lactic acid, active coal is separated on the filter press. The filtered 40% milk acid is drained into the collection of finished products, and from it is served on the packaging.
To obtain 70% acid, 40% lactic acid is re-evaporated with a large vacuum in vacuum devices. 70% milk acid is drained into the container, and then fed to the filter filter filter. The filtered acid is drained into the collection, from where it is fed or on the preparation of 70% pasty acid, which is obtained by the introduction of small amounts of chalk (4% to the mass of the acid). In this case, there is a partial substitution of ions H + lactic acid ion Ca2 + and about 10% acid turns into crystalline lactate, which binds milk acid.