Lactic acid is your ally! The most important source of energy or the culprit of fatigue and muscle soreness
Although anaerobic respiration for each glucose molecule, only two ATP molecules are formed, and with aerobic - 38 molecules, but in the first case, ATP synthesis is 2.5 times faster (anaerobic respiration produces five ATP molecules in the same period of time for which aerobic respiration produces two). Anaerobic respiration can therefore supply energy quickly. The glycogen stored in the muscles serves as a source of glucose. The energy extracted from it is enough for maximum muscle activity for 90 seconds.
All of these systems are working effective under regular load.
So, we see that the systems of phosphocreatine and anaerobic respiration supply energy quickly, but only for a short time. The aerobic system is able to serve as a source of energy indefinitely with a sufficient amount of the respiratory substrate. In sports that rely on a short and sharp increase in muscle activity, such as sprinting or lifting a barbell, energy is supplied mainly by the phosphocreatine system. When running 200 meters, anaerobic respiration can serve as an additional source of energy. When running 400 meters, it already supplies most of the energy, and in games such as tennis, squash or football, almost all the energy at the moment of maximum tension comes from this system. Endurance sports, such as marathons, jogging, and cross-country skiing, depend almost entirely on aerobic respiration.
Oxygen uptake during muscle exercise and during recovery.At the end muscle work oxygen consumption does not immediately return to the level characteristic of the resting state (0.25 l / min). During the recovery period, the person continues to breathe heavily for some time. The amount of oxygen consumed in this case is the oxygen debt. This oxygen is used:
1. To replenish oxygen in the body, i.e. to restore it normal level in the lungs, in tissue fluids, myoglobin and hemoglobin.
2. For the regeneration of phosphocreatine- at the end of muscle work, creatine reattaches phosphate; the energy for this is supplied by aerobic respiration.
3. Replenishment of oxygen supply in the body and the regeneration of phosphocreatine occur quickly; this is evidenced by the steeply descending part of the curve, corresponding to the first minutes of recovery (Fig. 9.10). The slower recovery (sloping part of the curve) is the period when lactic acid accumulated during anaerobic respiration is removed from the muscles. Lactic acid enters the bloodstream and is transported from the muscles to the liver, where it is oxidized to form pyruvic acid and reduced NAD. Some of this pyruvic acid enters the normal aerobic pathway through the Krebs cycle and undergoes oxidation to form ATP. This ATP can then be used to convert the rest of the pyruvic acid (about 75%) back to glucose in a process that is reverse glycolysis. In the heart muscle during heavy exercise, lactic acid can also turn into pyruvic acid, being oxidized by NAD, and this process serves as an additional source of energy here.
- Return to the section heading " "
Among bodybuilders (especially beginners), it is common to blame lactic acid for all problems. This is especially true for the occurrence of fatigue during training, in respiratory disorders, in convulsions, in the occurrence of burning sensations in the muscles, in pain that occurs in the muscles the next day. However, there is no scientific evidence that lactic acid somehow connected with all these negative consequences that often occur during the exercise. If such a connection exists, then it is accidental.
Major energy player
In fact, during the exercise, it is lactic acid that plays the main role in the processes of energy production by the human body. It is it that provides energy to the body, helps to use dietary carbohydrates for energy production, accelerates wound healing, and lactic acid plays a major role in the production of glycogen and glucose in the liver. This is critical to a successful workout! Lactic acid actually protects the athlete from all emerging stressful situations.
However, as with any process in the body, in addition to the undoubted advantages, there are also disadvantages. Lactic acid after production breaks down into hydrogen ions and lactate ions. Hydrogen ions are the acidic component of lactic acid. Most scientists believe that it is hydrogen ions that produce a change in electrical signals in nerves and muscles, after which a change in energy reactions occurs and muscle contractions are weakened. It is possible that the burning sensation in the muscles that is familiar to all bodybuilders occurs precisely because a large amount of hydrogen ions accumulate in the muscle tissue. And fatigue can initiate an excess accumulation of phosphate and potassium ions. And lactic acid actually prevents this accumulation.
Lactate is blamed for fatigue initiation only associatively. In the course of numerous experiments, scientists found that when performing exercise with high intensity, a large amount of lactic acid accumulates in the muscles and blood of a person. But the athlete's body, contrary to popular belief, is very fond of lactate. After all, in fact, this is the fuel that comes into action as quickly as possible. It is lactate that muscles and the heart prefer to use during exercise. Even during exercises that last several hours, lactate quickly and steadily supplies energy to the system.
Lactate is not an enemy, but a friend of all athletes. And when you learn more about lactic acid, the picture will be completely different. A bodybuilder can use the full power of lactic acid to give his body a lot more energy. You will not be familiar with such a word as overwork. After all, in fact, the benefits of lactic acid are much greater than the harm.
Lactic acid is not a dairy product
Lactic acid is formed in the body as a result of the breakdown of glucose. Glucose, sometimes called blood sugar, is the main source of carbohydrates. For the human nervous system and brain, this is a critically important type of fuel. For muscles during exercise, glucose is also important. Tissue cells break down glucose and produce adenosine triphosphate (ATP), which is the energy source for most of the chemical reactions that take place in human body. It depends on the amount of ATP how much time your muscles are able to work fully.
The process of formation of lactic acid in the body occurs without the participation of oxygen, so this process is also called anaerobic metabolism. The production of ATP associated with lactate is small, but occurs at an exceptionally high rate, which makes it ideal for covering the needs of the athlete's body for energy. Especially when the body works with an intensity of 65% of the maximum.
Whenever the body breaks down carbohydrates for energy, lactic acid is produced. The faster the body breaks down glucose and glycogen, the more lactic acid is produced. The body uses fats as fuel only when it is either at a complete rest or when the bodybuilder is working with submaximal weights. When the exercises are performed at an intensity of 65% (and almost all training programs are designed for just such an intensity), then the body mainly processes carbohydrates. The more an athlete consumes carbohydrates with food, the more lactic acid is formed in his body.
How lactic acid is involved in metabolism
The human body uses lactic acid as a chemical intermediary in the processing of dietary carbohydrates. Carbohydrates in the stomach are broken down and enter the bloodstream, and in the form of glucose enter the human liver. But the main amount of glucose does not enter the liver. Walking the path along big circle blood circulation, glucose enters the muscle tissue, where it is converted into lactic acid. Then it re-enters the bloodstream and moves to the liver, which uses lactic acid as a raw material in the formation of glycogen. This indirect route produces more glycogen than the direct route. When blood glucose is delivered to the liver. This goes to show how important lactic acid is in carbohydrate metabolism.
Many fibers, mainly muscle fibers, produce and use lactic acid all the time. The level of acid in the blood really reflects the balance between the production of acid and its consumption. If its level has increased, then this does not mean at all that the synthesis of lactic acid has increased in the body. It simply decreased its consumption in muscle tissue and its removal from the blood.
The amount of lactic acid produced as a result of synthesis equals the amount of carbohydrates that the body breaks down for energy. No matter how many carbohydrates an athlete uses, most of them will turn into lactate, which will then be used as fuel or transported to other body tissues. If a bodybuilder is exercising at a high intensity, then the production of lactic acid will accelerate. Since the athlete's body cannot fully use all the amount of lactic acid received, it "stores" it in the blood and muscle tissue. However, if the pace of exercise slows down or training stops altogether, the level of production will very quickly equal the level of consumption. Therefore, the bodybuilder who has learned to manage the amount of lactic acid in his own body will never experience problems with energy.
Fast fuel for high intensity exercise
During exercise, slow-twitch muscle fibers, the heart, and respiratory muscles prefer to use lactate as fuel. For example, when the intensity of exercise increases, the consumption of lactate by the heart muscle increases several times. And the use of glucose increases slightly. Heart muscle fibers do not need glucose - they use lactate to meet their energy needs immediately.
Lactic acid is a very fast fuel that athletes use to improve their performance. After carbohydrates have entered the stomach, the concentration of lactic acid and glucose instantly rises in the blood. Lactic acid is immediately removed from the bloodstream, so it high concentration short-lived. Blood glucose is converted by the body into lactate, which can be reused.
to be continued
I often ask my students - future sports doctors - a question about what they think about lactic acid. And their usual response: "Nothing good!" They blame her for everything from pain and muscle cramps to fatigue and injury. It is seen as a by-product to be avoided at all costs.
Did you know, I tell them, that lactic acid plays a major role in energy production during exercise? And it is not at all a harmful by-product of metabolism. It provides energy, promotes the absorption of carbohydrates, and serves as fuel for the liver to produce glucose and glycogen. In fact, lactic acid is a natural remedy designed to help our body cope with stressful situations. "But there is also a downside to the coin. When the body produces lactic acid, it breaks it down into a lactate ion (lactate) and a hydrogen ion. In lactic acid, it is the latter and is actually an acid.It interferes with the electrolytic signals of the nerves and muscles, slows down energy reactions and weakens muscle contractions.It is what causes that burning sensation that you feel during intense training.So if you feel tired, blame it on nothing else like a hydrogen ion.
But, as a rule, lactate is also blamed for the company. Although, in reality, our body is fine with it. It is an extremely fast fuel for the heart and muscles. Lactate plays a vital role in ensuring a stable supply of carbohydrates to the body, even during long hours of physical activity.
Lactate is a friend of all those who train with weights, a friend of football players, triathletes, long-distance runners, swimmers and cyclists. When you learn more about this substance, everything will appear in a completely different light. By appreciating the action of lactic acid, you can increase your energy level and defeat fatigue!
Lactic acid is truly the "royal" metabolite
Lactic acid is formed from the breakdown of glucose. Sometimes referred to as "blood sugar," glucose is our body's main source of carbohydrates. It is the main fuel for the brain and nervous system, as well as for the muscles during exercise. When glucose is broken down, cells produce ATP (adenosine triphosphate), which provides energy for most chemical reactions in the body. The level of ATP determines how quickly and for how long our muscles can contract when physical activity.
The production of lactic acid does not require the presence of oxygen, which is why this process is often referred to as "anaerobic metabolism". Many people believe that muscles produce lactic acid when they don't get enough oxygen from the blood. In other words, you are in an anaerobic state. However, scientists claim that lactic acid is also formed in muscles that receive enough oxygen. An increase in the amount of lactic acid in the bloodstream only indicates that the level of its intake exceeds the level of removal. Oxygen does not play a significant role here.
Lactate-dependent ATP production is very small, but has a high rate. This circumstance makes it ideal for use as a fuel when the load exceeds 50% of the maximum. During rest and submaximal exercise, the body prefers to break down fats for energy. At loads of 50% of the maximum (the intensity threshold for most training programs), the body is rebuilt to preferential consumption of carbohydrates. The more carbohydrates you use as fuel, the more lactic acid is produced.
metabolic mediator
The body uses lactic acid as a biochemical messenger for carbohydrate metabolism. Carbohydrates are digested and circulated from the intestine to the liver primarily in the form of glucose. However, instead of going to the liver to be converted to glycogen, most of the glucose from dietary carbohydrates bypasses the liver and goes directly to the bloodstream, reaches the muscles, and there it is converted into lactic acid. It, in turn, goes back to the blood, then to the liver, where it is used to create glycogen. Your body does not form most of its liver glycogen directly from blood glucose, but through the formation of lactic acid. Scientists call this process the "glucose paradox".
Many tissues, especially skeletal muscles, constantly synthesize and use lactic acid. Its blood level reflects the balance between production and consumption.
The production of lactic acid is proportional to the amount of carbohydrates broken down for energy needs in the tissues. When carbohydrates are consumed, a fairly large part of them is converted into lactate, which is then used by the same tissues as fuel or transported through the bloodstream to other tissues for energy purposes. The rapid use of carbohydrates as fuel, such as during intense exercise, accelerates the production of lactic acid. Temporarily, it begins to accumulate in the muscles and blood, because it cannot be used as a fuel very quickly. If you slow down your exercise or stop exercising altogether, lactate utilization will soon equalize with lactate production. Dr. George Brooks, professor in the Department of General Biology at the University of California, described the dynamics of lactic acid production and utilization in the metabolic process in his so-called "Lactate Shuttle Theory". It shows the leading role of lactic acid in carbohydrate metabolism and its importance as a fuel for metabolism. IN exclusive interview Dr. Brooks said: “Lactic acid is generally treated poorly. But if athletes could learn to control and use this chemical process, they could train harder and for longer. Regulating lactic acid levels is the key to success in high-intensity sports!"
The heart, slow twitch muscle fibers, and respiratory muscles prefer to use lactate as a fuel during exercise. In the heart, for example, its consumption increases significantly with increasing exercise, while the use of glucose remains unchanged.
Lactic acid is a very "fast" fuel, which can help athletes improve their performance. After ingestion of a high-carbohydrate meal, the concentration in the blood of both glucose and lactic acid increases. But the level of lactate rises slightly, as it is removed quickly enough. The body converts glucose (which does not move as fast in the blood) into lactate, so it reaches its target faster. Using lactic acid as a "middleman" helps get rid of dietary carbohydrates without raising insulin levels and stimulating fat synthesis. During training, you do not need this rise, as it reduces the availability of carbohydrates, which are essential for an intense metabolism.
Why is lactic acid so important in regulating metabolism? There is no exact answer yet, but there are certain physiological causes. Lactic acid, unlike glucose and other fuels, has a smaller molecular size, so it is easier for it to pass from one tissue to another. It penetrates cell membranes through an instantaneous process called "facilitated transport". Other fuels require slower transport systems such as insulin. Thus, lactate enters the cells and bloodstream faster and in greater quantities. Muscle cells with large glycogen stores cannot release significant amounts of the potential energy source glucose because they lack the key enzyme responsible for producing free glucose to release into the blood.
Lactic acid and fatigue
"Work until you burn!" your aerobics instructor says. Quite known fact that during intense physical exertion, lactic acid causes a burning sensation associated with muscle fatigue. Probably so. Hydrogen ions interfere with muscle contraction and energy-producing reactions.
During a workout nervous system protects the heart, brain and muscles from oxygen deficiency. The level of lactic acid in the muscles is an important signal for her in the distribution of blood throughout the body. When the system determines that the oxygen supply must be reduced somewhere, it reduces the blood flow there, which causes fatigue.
However, lactic acid is not responsible for all types of fatigue during exercise. During activities that require a lot of endurance, such as marathon running or triathlon, its blood level does not change, despite the fact that production increases. This is because the body's ability to produce it matches its ability to use it as fuel. At the beginning of the race, there is a significant increase in the level of glucose consumption by the muscles and the breakdown of glycogen. It's an uptempo carbohydrate metabolism causes an increase in the production of lactic acid and an increase in its content in the blood.
Once the blood is directed to the working muscles, you can "send" the lactate to other tissues for energy. At the same time, its level in the muscles and blood will decrease, although the body continues to produce it in large quantities. Often during a run or workout, you feel a sudden sense of relief. This feeling is called "second wind". Studies have shown that during exercise, the production and removal of lactic acid is 300-600% greater than at rest, even if oxygen consumption has stabilized at a submaximal level.
Muscle pain and cramps
Lactic acid is not the cause of pain and muscle cramps. The pain that appears in the muscles the day after training is caused by damage to the muscle fibers and their inflammation. Convulsions are caused by muscle receptors that are overexcited by muscle fatigue. Many athletes use massage, hot baths, and other relaxation techniques to remove lactic acid from muscle fibers to relieve pain and cramps. While such methods have their benefits, getting rid of lactic acid is not one of them. Lactate is used as fuel by the muscles during both training and recovery, rather than remaining in them like recycled motor oil.
Put Lactic Acid to Work for You
A properly designed training program that combines periods of high-intensity training with endurance training can speed up the removal of lactic acid. Fortunately, most training programs are built that way. Your body must learn to remove lactate quickly to be successful in competition.
Lactic acid metabolism helps you run, swim, or bike faster. To increase the body's ability to use lactate as a fuel, it is necessary to increase the level of lactate in the muscles during exercise. Workouts with great content lactate in your system stimulates the body to produce enzymes that accelerate its use. A number of studies have proven the importance of lactate content in sports drinks. Athletes learn to endure this so-called "burning". Scientists call this "habituation." Vince Lombardi, the immortal coach of the Greenbay Packers, once said, "When movement causes pain, pain causes movement." If he were a professor of physiology, his statement would sound like this: "When the level of lactate in the muscles rises, pain becomes a habit." Good thing he was a football coach.
With high intensity interval training the cardiovascular system adapts by increasing the supply of oxygen to muscle and other tissues. Therefore, you will have to break down fewer carbohydrates to produce lactic acid. In addition, better blood circulation helps speed up its delivery to tissues and removal from the bloodstream.
Endurance training induces muscle adaptation, which also speeds up the removal of lactic acid. Running, swimming or cycling cause the greatest development of microcirculation and functional capacity of mitochondria in skeletal muscle cells. With the increase in this ability, the use fatty acids as an energy source and thus reduces the formation of lactate. With an increase in the functional capacity of muscle mitochondria, the removal of lactic acid from the body also occurs faster.
Nutrition also plays an important role. Intense and hard training depletes glycogen stores in the muscles and liver. Therefore, all endurance athletes need a carbohydrate-rich diet.
Carbohydrates provide the fastest glucose supply, so the athlete feels great and has a source of fast energy. Moreover, glucose contributes to the replenishment of glycogen stores during the recovery period. When blood glucose and muscle glycogen levels are restored, glucose becomes a source of lactate formation, which helps replenish liver glycogen stores.
Lactic acid CH3CHOHCOOH is formed as a result of the anaerobic conversion of carbohydrates by lactic acid bacteria. Lactic acid is an organic monobasic hydroxy acid. The hydroxyl group of this acid can be in two (a and B) positions of the carbon chain. Therefore, two types of lactic acid are distinguished: a-hydroxypropionic CH3CHOHCOOH and B-hydroxypropionic CH2OHCH2COOH. Industrial value is a-hydroxypropionic acid produced in the process of lactic acid fermentation.
Lactic acid is widely used in the chemical, food, and pharmaceutical industries. In the USSR, under industrial conditions, food lactic acid is obtained by deep cultivation using the bacteria Bacterium delbruckii (synonymous with Lactobacillus delbruckii), which belong to homofermentative thermophilic bacteria with an optimum development of 48-50 °C. The production value of these microorganisms also lies in the fact that the temperature maximum for their development is in the range of 54-56°C, and intensive acid formation is provided at a relatively high temperature - 50°C. This temperature creates elective conditions. Most microorganisms do not develop in this case, since the indicated temperatures are far beyond the optimum and maximum for their development. The production of lactic acid includes the following main technological stages: lactic fermentation, treatment of the fermented solution and filtration, splitting of calcium lactate, evaporation of lactic acid.
The formation of lactic acid from glucose during fermentation by homofermentative lactic acid bacteria occurs according to the equation

The overall equation for the conversion of glucose into lactic acid using the enzyme system of lactic acid bacteria can be represented as follows:
The breakdown of glucose occurs along the FDF pathway, the bacteria has all the necessary enzymes for this, including aldolase. Hydrogen, split off during the dehydrogenation of triose phosphate, is transferred to pyruvate. The scheme of lactic acid biosynthesis is presented below.
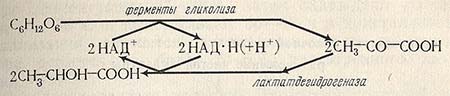
Another variant of the lactic acid fermentation scheme includes the breakdown of glucose to pyruvic acid and the reduction of pyruvic acid to lactic acid.
Lactic acid is an unstable chemical compound, and depending on the conditions of production and storage, it easily forms dehydration products called lactic acid anhydrides.
Lactic acid crystals melt rapidly at atmospheric pressure to form a colorless syrupy liquid. specific gravity 1.21 odorless with a sharply sour taste.
lactic acid fermentation
A variety of carbohydrates are used to produce lactic acid. In industry, acid is usually obtained from raw materials containing glucose, sucrose and maltose. Refined molasses, molasses, starch (corn, potato), previously saccharified with malt, can serve as such raw materials. The concentration of sugar in the fermented medium, depending on the type of raw material and fermentation conditions, can vary from 5 to 18%. For the fermentation of sulfite liquors, lactic acid bacteria of the species L. plantarum can be used. They ferment hydrolysates containing pentoses (xylose, arabinose) with approximately the same yield of acetic and lactic acids. The separation of these formed acids is carried out by the method of distillation from fermented solutions.
Under industrial conditions, lactic acid is obtained by a deep method using the culture of L. delbruckii. Molasses, sucrose, starch hydrolysates are used as the main raw materials. The concentration of sugar in the medium is 5-20%, pH 6.3-6.5. During fermentation, the pH of the medium is maintained with chalk, which is added 3-4 times a day. Lactic acid fermentation is carried out at a strictly constant temperature of 50 °C. Lowering the temperature to 46-48 °C causes a sharp weakening of the biochemical activity of the culture and contributes to the development of extraneous microflora. An increase in temperature, for example to 53-55 ° C, also causes inactivation of the culture and a slowdown in fermentation.

Biologically active substances have a positive effect on lactic acid fermentation. For this purpose, an extract from malt sprouts is added to the nutrient medium. During normal fermentation, bacteria ferment 1-1.5% of sugar per day, and the entire fermentation cycle ends in 7-11 days. The amount of unfermented sugar is 0.5-0.7%, and the concentration of calcium lactate is 10-15%.
Fermented liquor treatment and filtration
To separate chalk and colloids, the fermented solution is heated to 80–90 °C, and then treated with slaked lime to a slightly alkaline reaction and settled for 3–5 hours. To remove coarse suspension and solid particles, the settled layer of calcium lactate solution is decanted. The solution is pumped to the filter press. Filtration is carried out at a temperature of calcium lactate solution of 70-80°C through a preheated filter press. The resulting filtrate is evaporated to a concentration of 27-30%, then cooled to a temperature of 25-30 ° C and kept for 36-48 hours in the crystallizer. Crystallization is considered complete if no more than 5-6% of dissolved calcium lactate remains in the mother liquor.
Breakdown of calcium lactate
washed out cold water calcium lactate is separated in a centrifuge and melted. In order to protect lactate from charring, the splitting of calcium lactate with sulfuric acid with the release of free lactic acid is carried out at 60-70 ° C. This reaction proceeds according to the equation
To separate the iron ions, the resulting crude lactic acid is treated with yellow blood salt at a temperature of 65 °C. Prussian blue precipitates. Heavy metals and arsenic are precipitated with sodium sulfate and barium sulfide. Activated charcoal is used to free lactic acid from dyes. After treatment, the resulting mixture is filtered, and the gypsum precipitate is washed to extract the remaining lactic acid.
Evaporation of lactic acid
After cleavage of crystalline calcium lactate and subsequent processing, crude lactic acid of 18-20% concentration is obtained, which is evaporated to achieve a 40% concentration. Evaporation of the acid is carried out in vacuum apparatuses at a residual pressure of 10-15 kPa and a steam pressure of 0.2 MPa. Lactic acid stripped off to 40% is clarified with active carbon and treated with yellow blood salt. After clarification of lactic acid, active carbon is separated on a filter press. The filtered 40% lactic acid is poured into the collection of finished products, and from it is served for packaging.
To obtain 70% acid, 40% lactic acid is again evaporated at high vacuum in vacuum apparatus. 70% lactic acid is poured into a container and then fed to a filter press for final filtration. The filtered acid is poured into the collection, from where it is served for bottling or for the preparation of 70% pasty acid, which is obtained by adding small amounts of chalk (4% by weight of acid) to it. In this case, the H+ ions of lactic acid are partially replaced by Ca2+ ions, and about 10% of the acid is converted into crystalline lactate, which binds lactic acid.