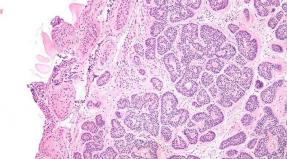
How the ethyl alcohol for fermentation is formed. Morphology bacteria. Alcohol fermentation
Glucose fermentation is one of the main reactions with which it is possible to prepare alcoholic beverages. It can be carried out by different paths, in each of which customized products are formed. This process plays a key role in many branches of our life, starting with cooking and preparation of wine-vodka products and ending with reactions occurring in our organism.
History
Ancient people used the process of fermentation of glucose and other sugars. They ate a little recruited food. Such food was safer, as it contained alcohol, in whose environment many harmful bacteria. In ancient Egypt and Babylon, people already knew how to ferment many saham-containing drinks and milk. When people at the end of the 18th century managed to explore this process, its types and opportunities of improvement, such industries such as KVASO, brewing and wine-vodka rose very highly qualitatively.
Types of fermentation
Oddly enough, this process is different. And distinguish the types of glucose glucose by final products. Thus, there is a lactic acid, alcohol, lemon-acid, acetone, oily acid and several other others. Let's talk a little about each form separately. Glucose lactic acid fermentation is the main process in the preparation of such products as the prokobivash, sour cream, kefir, cottage cheese. It is also used to preserve vegetables and performs a key function in our body: in conditions of lack of oxygen glucose turns into the final product - milk acid, which causes pain in the muscles at the time of workout and a little after it.
This process occurs under the influence of oxygen, and in general It can be recorded by the following equation: 2 ° C 6 H 12 O 6 + 3O 2 \u003d 2 ° C 6 H 8 O 7 + 4N 2 O. Before this type of fermentation discovered, people mined a citric acid exclusively filtering the fruit of the appropriate tree. However, in the lemon of this acid no more than 15%, so this method was inexpeded, and after the discovery of this reaction, all the acid began to receive the fermentation method.

Oily acid fermentation
Let us turn to the next type. This type of fermentation occurs under the action of oily acid bacteria. They are widespread, and the process they cause, plays a key role in biologically important cycles. Using these bacteria and decomposition of dead organisms. Oil acid formed during the reactions, attracts with its smell of padelvers.
This type of fermentation is used in industry. As it is easy to guess, they get oily acid. Her esters are widely used in perfumery and have nice smell, unlike her itself. However, not always oily-acid fermentation benefits. It can cause damage of vegetables, canned, milk and other products. But this may happen if only oil-acid bacteria got into the product.
We will analyze the mechanism of oily acid glucose fermentation. Its reaction looks like this: C 6 H 12 O 6 → CH 3 CH 2 CH 2 COOH + 2CO 2 + 2H 2. As a result, the energy is also formed, which ensures the vital activity of oily acid bacteria.

Acetone-butyl fermentation
This type is very similar to oily acids. It can be wandering in this way that not only glucose, but also glycerin, and peyrograd acid. This process can be divided into two stages: the first (sometimes called acid) is actually oily acid fermentation. However, in addition to oil, acetic acid is also distinguished. As a result of glucose fermentation in this way, we get products that go to the second stage (acetone bututyl). Since all this process is also occurring under the action of bacteria, then when acidifying the medium (increasing the concentration of acids), special enzymes are selected by bacteria. They induce the reaction of transformation of glucose fermentation products in and acetone. In addition, some ethanol may be formed.
Other types of fermentation
In addition to the listed five types of this process, there are several more. For example, it is acetic fermentation. It also occurs under the action of many bacteria. This type of fermentation can be used for useful purposes when marinated. He protects food from pathogenic and dangerous bacteria. It is still distinguished by alkaline or methane fermentation. In contrast to previous types, this type of fermentation can be carried out for most organic compounds. As a result of a large number of complex reactions, organic substances are split into methane, hydrogen and carbon dioxide.

Biological role
Frying is the most ancient way to produce energy by alive organisms. Some creatures produce organic substances, passing energy, while others destroy these substances, while yielding energy. This built our whole life. And in each of us, fermentation flows in one or another. As we said above, lactic acid fermentation occurs in muscles with intense training.
If you are interested in the biochemistry of this very interesting process, it is worth starting with school textbooks in chemistry and biology. In many university textbooks, there is so detailed material that after reading them you can become an expert in this area.
Conclusion
So we came to an end. Disassembled all types of glucose fermentation and general principles The flows of these processes that play a very important role in both the functioning of living organisms and in our industry. It is possible that in the future we will open a few more species of this ancient process and learn how to use them for yourself, as you have done with already known to us.
Alcohol fermentation
| Name of parameter | Value |
| Theme of article: | Alcohol fermentation |
| Rubric (thematic category) | Education |
Alcohol fermentation cause various microorganisms. More often this is the yeast of childbirth Saccharomyces., Mushrooms Rodov Oidium., MUCOR., Monilia.. Can cause fermentation some bacteria Clostridium., as well as representatives of the family Enterobacteriaceae.. For most microorganisms, alcohol is a by-product and only for yeast Saccharo-Myces. - ϶ᴛᴏ The main final fermentation product.
Yeast is widespread in nature. ʜᴎʜᴎ are found on the surface of plants, in the nectar of flowers, in the water bodies, the digestive tract of man and animals, in the soil. In the process of evolution, yeast adapted to habitat in various places containing carbohydrates. It is all so-called, "wild yeast." Culture of yeast used in the food industry is separated by long-term detection from wild yeast.
The main pathogens of alcohol fermentation yeast-sugaromycetes are optional anaerobes. In the anaerobic conditions, the energy required for life activity is obtained by fermentation of mono- and disaccharides, and in the presence of air oxygen - due to aerobic breathing. From the combustion sections, the yeast is better used hexoses. Some species grow well on media with penos. Polysaccharides are used yeast only after pre-hydrolysis. As a source of nitrogen, the yeast is usually used ammonium salts, amino acids, peptones, less often nitrates and nitrites. Yeast amino acids synthesize independently, in connection with this, ammonium salts are the only sources of nitrogen. It is worth saying that for normal vital activity, yeast needed in phosphorus (for the synthesis of proteins and coenzymes), in potassium and sodium salts. With a lack of sodium salts, the kidding of yeast is delayed, and in the absence of these salts, yeast is not growing. Some yeast need vitamins, others are capable of all necessary vitamins to synthesize themselves. Microelements (dry, copper, cobalt) increase the activity of yeast enzymes.
The optimal temperature of the development of most types of yeast is about 28-30 ° C, but the temperature range is quite wide - from 0 0 s (even -7 0 s) to 48-50 0 C. Most yeast grows in the pH borders from 3.0 to 8 , 0, the optimal pH value of 3.5-6.5.
The first form of fermentation along Neubourg.Before the beginning of alcohol fermentation of Oligosahara, at first hydrolyzed by the corresponding yeast enzymes to hexose. Next, the glycolithic method is carried out by the cleavage of hexosis and the formation of PVCs. Under the influence piruvatdecarboxylase Microorganisms from PVCs are cleaved from 2 and acetaldehyde is formed:
EMP-POD PDC
C 6 H 12 O 6 → 2 CH 3 SosoPon → 2 CH 3 SNO + 2 CO 2
Acetaldehyde serves as a finite hydrogen acceptor. It is restored to ethanol with the participation of the enzyme alcoholdhydrogenase. Frying implies strict equilibrium of oxidation and recovery processes. For this reason, over, restored at one of the stages of fermentation, should oxide at another stage. Oxidation Nadn 2 occurs simultaneously with the restoration of acetaldehyde to ethanol:
2 CH 3 SNO + 2 NADH 2 → 2 CH 3 CH 2 it + 2NV +
Τᴀᴋᴎᴍ ᴏϭᴩᴀᴈᴏᴍ, with alcohol fermentation, the main product of conversion of sugars is ethanol and CO 2. Such a Neberd process called the first form of fermentation (Figure G.1). The total reaction of the first shape of fermentation: from 6 H 12 O 6 → 2 CH 3 CH 2 it + 2 CO 2
Alcoholic fermentation flows most intensively in an acidic medium (pH 4.0-4.5), at a temperature of 30 0 s and a concentration of sugar 10-15%. Increased sugar concentration leads to a slowdown, and then discontinuation of the process. Alcohol fermentation is an endogenous process. Sugar is adsorbed on the surface of the yeast cell, penetrates inside and metabolized by enzymes. The resulting alcohol and CO 2 diffuse from the cell into the environment. With an increase in sugar concentration, the osmotic pressure in yeast cells increases, plasmolysis occurs, due to which the alcohol and metabolic processes are absorbed in the cells.
The second form of fermentation along Neubourg.The progress of fermentation can change significantly on the basis of specific conditions. If in the culture of wandering yeast add sodium bisulfite, he connects acetic aldehyde. So, acetaldehyde is blocked and excluded from the subsequent process:
CH 3 SNO + NaHSO 3 → CH 3 SNONSO 3 NA
Under such conditions, the hydrogen acceptor torn off from NAPH 2 is phosphoglycerin aldehyde, which turns into glycerin-3-phosphate, and then dephosphorilated with formation glycerin. Total reaction of the second shape of fermentation:
C 6 H 12 O 6 → C 3 H 5 (OH) 3 + CH 3 Sonya 3 Na + CO 2
The total amount of synthesized ATP with this fermentation form is zero and, therefore, the process cannot provide the growth of cells, but it is used in industry to obtain glycerol. Glycerin is used in confectionery, perfume and other industries.
The third shape of the fermentation of neubourg.A similar variant of alcohol fermentation occurs when the medium is ledgering (adding NAHSO 3 or Na 2 HPO 4). Under these conditions, acetaldehyde is oxidized by over-dependent dehydrogenase into acetic acid. Formed at this stage of NADP 2 is used to restore the equivalent number of acetaldehyde in ethanol. At the same time, NAPH 2, obtained during the oxidation of 3-phosphoroglycerin aldehyde, is used to restore phosphoglycerol aldehyde to glycerol-3-phosphate, which is then converted to glycerin. Total reaction of the third shape of fermentation:
2 C 6 H 12 O 6 + H 2 O → 2 C 3 H 5 (OH) 3 + CH 3 CH 2 OH + CH 3 Soam + 2So 2
Such chemistry of the process is favorable for cells, since the formed acetic acid reduces the pH of the medium, after which normal alcohol fermentation renews again.
Alcohol and glycerin, or glycerinpirograde (HPVK), fermentation are closely connected. IN initial stage Alcohol fermentation appears glycerin. This is explained by the presence of a kind of induction period at the beginning of fermentation, ᴛ.ᴇ. time is extremely important for the accumulation of acetaldehyde. ᴀᴋᴎᴍᴀᴋᴎᴍ ᴏϭᴩᴀᴈᴏᴍ, at the beginning, the GPVK fermentation prevails. But even during the period of stormy alcohol fermentation, along with alcohol, other products are formed.
Pasteur effect.In the presence of molecular oxygen (in aerobic conditions), the yeast quickly switches from fermentation to aerobic breathing. In this case, the PVC, formed from glucose and other substrates, is oxidized through the Krebs cycle to CO 2 and H 2 O. At the same time, the cycle of tricarboxylic acids (CTC) provides cells by a number of metabolites necessary for further biosynthetic reactions. In energy terms, breathing is more profitable than fermentation. For this reason, in aerobic conditions, yeast grow better and form a large biomass.
Suppression of fermentation in aerobic conditions is called pasteur EffectSince Pasteur first established that molecular oxygen reduces the formation of ethyl alcohol and CO 2, but contributes to the active breeding of yeast cells. This effect is used in yeast production, where it is extremely important that sugar consumed to reproduce the yeast to accumulate their biomass.
CrabrTree effect.Alcohol fermentation can occur in conditions of considerable aeration at high glucose content in the medium. Suppression of aerobic respiration at a high concentration of glucose (high speed of her assimilation) is customary to be called crubri effect, or catabolite repression. This effect is not observed when growing yeast on media containing less digestible sugars. Catabolic repression of aerobic respiration not only reduces the preparation of energy yeast, but suppresses the biosynthesis of intermediate TSK products and the glyoxylate cycle. In such conditions, the acids necessary for biosynthesis acid are formed by carboxylation of PVCs with the participation of the enzyme piruvatakarboxylase:
CH 3 SosoPon + ATP + CO 2 + H 2 O → Sosoons 2 Soam + ADF + F N
Alcohol fermentation is the concept and types. Classification and features of the category "Alcohol fermentation" 2014, 2015.
Alcohol fermentation is the process of converting sugar in anaerobic conditions in carbon dioxide and ethyl alcohol:
From 6 H 12 O 6 - "2 2 + 2C 2N 5
Ethyl alcohol is one of the widespread sahara fermentation products by microorganisms. Even plants and mushrooms in anaerobic conditions accumulate ethyl alcohol.
Chemistry of alcohol fermentation.
The causative agents of alcohol fermentation are yeast, which are grown in aerobic conditions, selecting the appropriate races with the necessary properties for this production.
The process of alcohol fermentation is carried out with the same energy reserve in the form of ATP and the same enzymatic way as glycoliz, up to the formation of peer's acid. The transformation of the pyruse acid into ethyl alcohol occurs in two stages. First, the pyruvate (peyrogradic acid) is decarboxylated by pyruvatdecarboxylase with the participation of thiaminepyrophosphate to acetaldehyde, and then acetaldehyde is restored to alcohol dehydrogenase in ethanol with NADH2 participation.
At the same time, the yeast receive energy to develop biochemical processes in a cell: glucose - "ethyl alcohol + CO 2 + 166 kJ / mol.
From an energy point of view, fermentation - the process is ineffective. So, if with oxidation of 1 grams of glucose molecule to CO 2 and H 2 O in the process of aerobic respiration, 36 mol ATF is synthesized, then in the process of alcohol fermentation - only 2 mol ATP.
Yeast can switch one type of metabolism (aerobic) to another (anaerobic).
Along with the main fermentation products - ethyl alcohol and CO 2, by-products are formed: glycerin, acetic aldehyde, acetic acid, succinic acid, as well as so-called seawous oils. The composition of the seawous oils includes propanol, 2-butanol, 2-methylpropanol, amylovy (pentanol) and isoamyl (trimethylbutanol) alcohols, which are products of normal ferry and yeast metabolism and detectable when growing yeast on any media. The synthesis paths of these substances have not yet been studied.
Higher alcohols are involved in the formation of aroma and flavors of alcohol fermentation. Yeast can be fermented in addition to glucose and peer-grade acid. As an intermediate product, acetaldehyde is formed when fermented If you add bisulfite ferrozham, then a new product is glycerin, however, the output of ethyl alcohol and CO 2 decreases.
The fermentation in the presence of bisulfite began to use in industry in the production of glycerol.
Many factors affect the conditions of alcohol fermentation: the chemical composition of the fermentable medium, i.e. its usefulness, concentration and acidity of the medium, the content of alcohol, temperature, the presence of extraneous microorganisms.
Most yeast are able to ferment monosaccharides, and from Disaccharides - Sakharozo and Maltose. Pentoses are fermented only by some yeast. Yeast cannot ferment starch, as they do not form amylolytic enzymes.
The most favorable sugar concentration in the medium for most yeast is 10-15%. With increasing sugar concentration, fermentation energy decreases, and at 30-35% sugar, fermentation usually stops.
The fermentation energy is called the ability of a certain amount of yeast to ferment in a certain period of time or another quantity of sugar. In nature, there are yeasts that can cause sachara fermentation even at a concentration of its 50-60% and higher.
A good source of nitrogen for most yeast is ammonium salts, but yeast can also use amino acids and peptides.
Frying usually flows in an acidic medium at pH 4-5. IN alkaline environment As a result of fermentation, glycerin is formed.
The highest fermentation rate is observed at a temperature of about 30 ° C; At a temperature of 45-50 ° C, fermentation stops as a result of the death of yeast cells. The decrease in temperature leads to a slowing down of fermentation, but it does not completely stop even at temperatures below about ° C.
Ethyl alcohol formed in the fermentation process adversely affects yeast. The accumulation of alcohol yeast at a concentration of 2-5% depending on the type and race of yeast acts on them in depressing. In most cases, fermentation stops when accumulated by yeast 12-14% (volume) alcohol. Removed by the seeders of the race of yeast, resistant to accumulation of 16-18 and even 20% alcohol.
The use of alcohol fermentation underlies the production of ethyl alcohol, beer, wines and bakery yeast.
To obtain ethyl alcohol, various raw materials of the three main groups are used: sugar containing sugar (sugar beets, feed pattern, or molasses, sugar cane, fruit juices); starch containing starch (potatoes, earthwood pear, corn, barley, oats, rye, wheat); Cellulose containing pulp (wood and sulfite liquor). Raw materials use depending on economic opportunities; It should be cheap and sufficient.
In the malt, in addition to amilates, proteolytic enzymes are also contained, which cause partial conversion of protein substances into soluble nitrogen-containing substances. The result is a wort, rich in both suphenic substances and other nutrients for yeast. Additional power supplies are made. This is always done by the recipe for each of this production.
Yeast is made to the resulting wort, the rassels of Saccharomyces Cerevisiae are most often used, which quickly multiply, alcohol resistant, possess high fermentation energy. There are other industrial crazy yeast.
At the end of fermentation, yeast is separated from fermented congestion, and the alcohol is distilled off on special distillation devices.
It turns out a raw alcohol and there remains production - bard, which is used to obtain feed yeast. Exhaust yeast are also used in the form of liquid and dry fodder yeast.
The raw alcohol is used both for technical purposes and for further purification - rectification. Microorganisms in the raw alcohol can get from air, raw materials, equipment. They can be lamp bacteria And wild yeast, which are capable of developing in anaerobic conditions in the presence of alcohol. They use nutrients of the medium, inhibit yeast products of their exchange, while the alcohol yield is reduced.
Alcohol is used in medicine, the production of various alcohol drinks. In recent decades, alcohol is treated as promising fuel. Production of alcohol on fuel has been established in the USA, Japan, Germany, in France, in Sweden, Australia and other countries. In some countries (USA, Brazil), the factories are ~ 200 thousand l per day. Alcohol is a raw material for the chemical industry. With a decrease in oil reserves (many scientists believes) alcoholic be comes to replace the petrochemistry (chemistry of bioethanol).
The main raw material for receiving beer is a barley malt, which is obtained from germinated barley grains (location). The malt amylases split the starch contained in it into simpler carbohydrates - maltose and dextrin, and proteases partially convert the protein contained in nitrogenous compounds digestible with yeast.
Non-skinned barley, rice, corn flour, water, hops, and beer wort are added to the malt. Beer wort is a full-fledged nutrient medium for yeast. Hop substances have an antibacterial effect and give beer specific bitterness and aroma.
To obtain most varieties of beer, it is predominantly lower fermentation yeast (Saccharomyces Carlsbergensis race), for some varieties of beer - saccharomyces cerevisiae. The fermentation caused by lower yeast proceeds slowly and calmly, lead it at relatively low temperatures - from 5 to 10 ° C. Gas is highlighted gradually, the foam is formed less, the yeast is not taken to the surface of the fermented medium and quickly settle on the bottom of the fermentation containers.
The fermentation caused by horse yeast proceeds violently and quickly at a temperature of 20-28 ° C. A lot of foam is formed on the surface of the wandering fluid. Under the action of the released CO 2, the yeast is taken into the upper layers of the substrate.
At the end of fermentation, the yeast settled on the bottom of the fermentation containers with a loose layer. The process of obtaining beer goes in two stages, i.e., two periods of fermentation are carried out - the main thing and reducing.
In the process of main fermentation at a temperature of from 6 to 10 ° C, the yeast is actively multiplied and sugar fermented intensively. Get the so-called green (immature) beer. It is poured with yeast sediment and directly at a reducing at a temperature of about 1 ° C. The yeast remaining in green beer, almost do not multiply and slowly ferment the remaining sugar. Carbon dioxide accumulates, 3-6% (by weight) of ethyl alcohol, coat products of fermentation: higher alcohols, organic acids, diacetyl, ethers. All of them take part in the formation of taste and flavor of beer.
Ripened beer brightened and directed to bottling, and yeast is removed by filtering or centrifuging. Chemical composition and the taste properties of different beers depend on the raw materials used, used races of yeast and production technology.
A piece of yeasts that have been on the bottom of the fermentation containers are again used to obtain beer, part are produced in the form of liquid or dry beer yeast as a product rich in vitamins B1, B2, B6, PP, pantothenic acid, and part goes on animal feed.
Beer is a perishable product. To increase the storage period of beer, it is pasteurize, sometimes chemical preservatives (sorbic acid, yuglon) are added, processing microwave.
To obtain wine, the feedstock serves grape juice, as well as fruit-berry juices. All juices are a good nutrient medium for various microorganisms. To get rid of the harmful microflora and from wild yeast, the juices are sulphitis (treated with sulphonic anhydride SO 2), and then subjected to fermentation. Sulfurian anhydride plays the role of both antiseptics and an antioxidant. It binds oxygen, while the redox potential of the medium decreases, which prevents the development of aerobic microflora and favors fermentation.
The fermentation of the juices for the manufacture of wines is carried out using pure wine yeast crops SACCH. (ELLIPSOIDEUS), and for sherry wine, also SACCH. Oviformis. To obtain certain wines, the production races of yeast choose the following requirements: they must completely select the wort, be resistant to increased content Sugar and alcohol, to SO 2, to the low pH quickly settle after fermentation and give a dense sediment. Different races SACCH. VINI, selected for definite) types of wines, have different temperature optimum fermentation, form a different amount of alcohol (10-18%), different side products, which is reflected in the taste and aromatically) wine properties.
To protect wines from microbial damage, they are pasteurize them, antiseptics (SO 2, sorbic acid) are introduced, treated with ultrasound, ultraviolet.
On all the fermentation industries, the quality and output of the finished products are largely dependent on the overall sanitary condition of production. All enterprises should support strict sanitation regime, as well as constant microbiological control at all stage) of the production process, including premises, containers, etc.
Bakery yeast (SACCH. CEREVISIAE) pressed and dry for the production of bakery products are obtained on specialized yeast factories. When producing bakery yeast, purified and diluted beet melassia is used as a nutrient medium - the waste of beetroot production. The beet melasse containing everything necessary for yeast is additionally added nitrogen and phosphorus-containing salts. Yeasts multiply at a temperature of about 30 ° C, pH 4.5 - 5.5, with continuous aeration. Yeast breathe and actively multiply, and do not roam. Most sugar are used to the synthesis of cell substances.
The gross mass of yeast is separated from the medium, washed with water, thicken and pressed to the content of the moisture of 73-75%. The resulting yeast mass is formulated in the form of briquettes with a content of yeast cells from 8 to 12 billion in 1 g. Briquettes are packaged in paper and cooled to a temperature of 4 ° C. Dry yeast is produced by a humidity of 8-10%.
The used races of bakery yeast should be well multiplied, possess high ferotic activity, be resistant when stored in pressed form and during drying. Currently, the following races of bakery yeast are used: Kievskaya 21, Odessa 14, Hybrids No. 176 and 196-6. Keep pressed yeast in the cold.
bacterium cage alcohol fermentation
Alcohol fermentation is the process of oxidation of carbohydrates, as a result of which ethyl alcohol is formed, carbon dioxide and energy is distinguished.
In 1836, the French scientist Canyar de la Tour found that alcohol fermentation is associated with the growth and reproduction of yeast. The chemical equation of alcohol fermentation was given by French Chemists A. Lavoisier (1789) and J. Gay-Loursak (1815). L. Paster came to the conclusion (1857) that alcohol fermentation can cause only living yeast in anaerobic conditions ("fermentation is life without air"). In contrast to this, the German scientist Y. Libih persistently insisted that fermentation occurs outside the living cell. The possibility of inflexible alcohol fermentation for the first time (1871) was indicated by the Russian biochemist M. M. Manassein. German chemist E. Buchner in 1897, ticking under a large pressure of yeast, arranged with quartz sand, received a cell-free juice, fermenting sugar with alcohol and CO2. When heated to 50 ° C and above, the juice lost the fermentation properties. All this indicated the enzymatic nature of the active principle contained in yeast juice. Russian Chemist L. A. Ivanov discovered (1905) that phosphate added to yeast juice increased several times the fermentation rate. Research of domestic biochemists A. I. Lebedeva, S. P. Kosticheva, Ya. O. Parnas and German biochemists K. Neibrega, Embued, O. Meyergood, etc. confirmed that phosphoric acid participates in the most important stages of alcohol fermentation. This type of fermentation has the greatest nationality.
Alcohol fermentation is the process of decomposition of sugar on alcohol and carbon dioxide. It proceeds under the action of microorganisms in the form of the following reaction:
C6N12O6 \u003d 2C2N5On + 2So2 + 27 kcal
Sugar Ethyl carbon dioxide
Alcohol Gas.
In addition to ethyl alcohol and carbon dioxide, at the same time by-products are also obtained: acetic aldehyde, glycerin, sevous oils (butyl, isobutyl, amyl and isoamyl alcohol), acetic and amber acid, etc.
The alcohol fermentation of carbohydrates is caused by yeast, individual representatives of Mukorovy mushrooms and some bacteria. However, mushrooms and bacteria produce alcohol significantly less than yeast. Only carbohydrates can be lost, and still very selective. Yeasts are fermented only by some 6-carbon sugar (glucose, fructose, mannose).
Schematically, alcohol fermentation can be depicted by the equation
C 6 H 12 O 6 → 2C 2 H 5 H + 2CO 2 + 23,5 + 104 J
glucose → Ethyl alcohol + carbon dioxide + energy.
The process of alcohol fermentation is a multistage, consisting of a chain of chemical reactions. The conversion of glucose to the formation of peyrogradic acid occurs in the same way as with breathing. These reactions occur without oxygen participation (anaerobo). Then the way of breathing and fermentation diverge.
With alcohol fermentation, peerogradic acid turns into alcohol and carbon dioxide. These reactions proceed into two stages. First, C02 is cleaved from the piruvata and acetic aldehyde is formed; The then acetic aldehyde joins hydrogen, restoring ethyl alcohol. All reactions are catalyzed by enzymes. In the restoration of aldehyde participates over-H2. Usually with alcohol fermentation, in addition to the main products, side is formed. They are quite diverse, but are present in small quantities: amylovy, butyl and other alcohols, a mixture of which is called a fusion oil - a compound from which a specific flavor of wine depends. The formation of side substances is due to the fact that the transformation of glucose is partially in other ways. The biological meaning of alcohol fermentation is that a certain amount of energy is formed, which is intensified in the form of ATP, and then consumes all the vital cell processes.
Alcohol fermentation is used by a man with deep antiquity in the manufacture of wine, beer, braghi, etc. The reason for fermentation began known only in the middle of the XIX century, after Paster found that the decomposition of sugar on alcohol and carbon dioxide is associated with the breath of yeast in anaerobic Conditions. Sugar fermentation is a complex biochemical process, therefore the equation above above expresses it only in a total of total.
The yeast, depending on the conditions of fermentation, form different amounts of fermentation products, among them either ethyl alcohol and carbon dioxide, or glycerin and acetic acid may prevail. Moreover, they do not ferret all sugar, but only monosaccharides (for example, glucose) and disaccharides (for example, maltose). Polysaccharides (starch) yeast is not able to ferment, as they do not have the enzyme (amylases) for splitting polysaccharides.
The fermentation depends not only on the conditions in which it proceeds, but also on the type and race of the used yeast. These conditions include sugar concentration, medium acidity, temperature and amount of accumulated alcohol.
The most favorable sugar concentration in the faster substrate for most yeast is about 15%, with higher concentrations, fermentation slows down, and then stops at all. However, some yeast can cause fermentation and in the content of a sugar in the sugar environment. At sugar concentration in the substrate in an amount of less than 10%, fermentation proceeds very sluggish.
Normal for alcohol fermentation is an acidic medium with a pH equal to 4 or 4.5. In an alkaline medium, fermentation proceeds with the formation of glycerol and acetic acid.
The best fermentation temperature is in the range of 28-32 ° C. At higher temperatures, fermentation slows down, and at 50 ° C it stops. The decrease in temperature reduces fermentation energy, although it does not fully stop even at 0 ° C. In practice, fermentation processes are conducted at temperatures in the range of 20-28 ° C with the roof fermentation and within 5-10 ° C with lower fermentation.
The rigging fermentation proceeds very energetically, with the formation of a large amount of foam on the surface of a substrate and with a rapid separation of carbon dioxide, whose streams are treated in the upper layers of the substrate. Yeast causing such fermentation is called horse yeast. After the end of fermentation, they settled on the bottom of the fermentation vessels.
The lower fermentation caused by lower yeast is much calmer, with the formation of a small amount of foam. Carbon dioxide is highlighted gradually and yeast remains in the lower layer of the substrate of the substrate.
Hinda yeast is used to obtain alcohol and bakery yeast, lower - for the production of wine and beer. High yeast is sometimes used to obtain wine and beer.
The alcohol formed in the fermentation process has a harmful effect on yeast. When accumulated in the substrate of alcohol, more than 16% to the volume of the substrate itself, fermentation stops, and the oppressive action of the formed alcohol begins to manifest itself at a concentration of 2-5%. Some races of specially accustomed yeast are able to withstand very high alcohol concentrations - up to 20-25%.
Alcohol fermentation normally proceeds in the anaerobic conditions created in the process of fermentation itself. But since yeast is optional anaerobas, they can decompose sugar and in aerobic conditions with the formation of carbon dioxide and water. It is noted that in the face of good aeration, the yeast reproduces hard. Therefore, in the production of bakery yeast, the rubbing substrate is blocked by air.
For industrial obtaining alcohol, starch-producing products are used as raw materials - potatoes, grain crops, as well as sozing of sugar production. Due to the fact that yeast is not able to ferment the starch, it is pre-sacrificed with a malt containing an amylase enzyme. The malt is obtained from sprouted barley grains. Currently, a mushroom malt (mushrooms of Aspergillus genus) is also used to precipitate, which is more profitable than barley malt in many respects. As a result of starch precipitation, Disaccharide Maltose is formed - malt sugar.
Using alcohol fermentation for food production
For the production of beer, the barley is most often used, from which malt is obtained, and a wedge-sugar liquid is prepared from malt, subjected to fermentation. The taste features of beer depends on the quality of raw materials, technology and yeast used. Low yeast used in brewing lead slow fermentation, do not cause significant cloudy wort, and at the end of the fermentation form a dense sediment at the bottom. Among the lower yeast there are severe-complete and weak yeast.
In the winemaking until the last time, the yeasts did not play the advantageous role that falls on their share in the production of beer. The bulk of the wines was obtained by self-protected the wort with the help of random yeast, located on the berries of grapes. The use of pure cultures in winemaking makes it possible to quickly and fully fermenting grape wort and get wine with a good bouquet. Separate races of wine yeast when saving grape wort are able to accumulate up to 10-14% alcohol. Each winery area has races of yeast, specific for this locality, so the variety of the obtained wine is determined not only by the variety of grapes and technology, but also the biological features of the yeast used.
Clean crops of yeast are necessarily used in the manufacture of effervescent wine. In the production of fruitful wines for each type of fruit or berries, the corresponding races of wine yeast are selected, which allows to obtain high quality wines.
Bakery yeast is used to obtain bread tests, which have a good lifting force and the ability to quickly multiply. The alcohol and carbon dioxide generated in the fermentation process are barely and raised the dough, and the side products of fermentation give the bread a special taste and aroma.
In the production of bread applied extruded and liquid yeast, as well as frivors. Pressed yeasts are a perishable product and therefore should be stored at low temperatures. An impurity in pressed yeast of wild yeast and bacteria indicates their low quality.
Liquid yeast is made directly on the bakeries. Unlike pressed yeast, they contain lactic acid bacteria. Generating milk acid, lactic acid bacteria prevent the development of a potato stick in the dough, causing a dying disease of bread.
Exquisites are the dough left from the previous baking. They are used to break the rye test. Rods contain yeast and lactic acid bacteria. In the medium of cultural yeast, which are used in production, extraneous microorganisms, causing damage to products, can fall. So, wild yeast is often pests of wine and beer. They change the taste and smell of these products, cause them to be clouding. Especially dangerous film yeast micoderma. Developing in wine and beer, they oxidize alcohol to carbon dioxide and water and give drinks an unpleasant taste.
Micoderma causes harm in the production of bakery yeast. The process of obtaining bakery yeast leads with the blowing of the substrate by air, as this contributes to their rapid reproduction. Micoderma in such conditions develops faster than real yeast. Since the micoderma does not have the ability to raise the dough, the presence of it in cultural yeast sharply reduces their bakery properties.
The pests of fermentation productions are also some types of lactic acid bacteria that cause clouded wine and beer. Separate representatives of spherical bacteria (pediococci) are able to give the beer a special taste and turbidity, and sometimes to endure it. Acetic acid bacteria can cause wine damage as a result of alcohol oxidation into acetic acid.
With alcohol fermentation, microorganisms turn carbohydrates with the formation of ethyl alcohol as the main fermentation product:
C6 H12O6 \u003d 2SH3CN2E + 2SO2
Some yeast include alcohol fermentation causative agents, mainly from the genus Sacchaomyces (S. Cerevisiae, S. Globosus, S. Vini, etc.). In small quantities, the alcohol can accumulate in an environment containing carbohydrates, with the development of some mushrooms from generics of Mucor and Fusarium and bacteria (Zymomonas Mobilis, Sarcina Ventriculi, Erwinia amylovora, etc.).
When accessing the oxygen, the yeast correctioning, It is beginning to oxidize carbohydrates, that is, fermentation transfers to the process of aerobic breathing. In this case, the coefficient of use of carbohydrates increases. Therefore, to obtain a greater mass of yeast, for example, in the production of bakery yeast, a nutrient medium in which their reproduction occurs, aerated.
On the contrary, in the production of alcohol, the process is conducted in anaerobic conditions.
Fishing sugars yeast with the formation of ethyl alcohol and CO2 goes along the path of Embued - Meyergood - Parnassa. In addition to ethyl alcohol, the so-called seawous oils are formed in the process of alcohol fermentation - amyl, isoamyl, butyl, isobutyl and other alcohols, which are by-products of the exchange of some amino acids - isoleucine, leucine and valine.
Usually, alcohol fermentation occurs at an acidic reaction of the medium (pH 4-5). If the reaction of the nutrient substrate is maintained at the alkali level (pH about 8), then glycerin will be one of the main fermentation products. In this case, alcohol fermentation is expressed as follows; By equation:
2С6Н12O6 + H20 - \u003d CH 3SOON - CH3CN2On + 2CN2On2on + 2С02
An even more sharply increases the yield of glycerol if fermentation flows in the presence of sodium sulfite Na2S03. At the same time, acetic aldehyde is associated with sulphite and cannot be restored with hydrogen into ethyl alcohol. An intermediate compound of hydrogen is an intermediate compound of dioxiacetone phosphate, which turns first into phosphoglycerin, and after cleaving the phosphate group, glycerin is formed.
In some cases, it is advisable to obtain glycerin and amyl alcohol with alcohol fermentation. Such production was carried out practically.
Not all sugars are fermented by yeast. Hexoses are usually well absorbed, but pentoses can assimilate only a very limited number of yeast species. It is not bad to be used yeast of disaccharides, but each type of these microorganisms is capable of assimilating only a strictly defined set. Pepper fermentation of more sophisticated sugar under the influence of yeast cell enzymes disintegrate on monosaccharides.
Some yeast can absorb simple dextrins, but the starch does not ferment. Only after preliminary precipitation with malt (or other methods) starch becomes suitable for alcohol fermentation. Many plants for alcohol fermentation are used by fiber, pre-exposed to acid hydrolysis.
In aerobic conditions, the yeast is capable of oxidizing organic acids and other connections. As a source of nitrogen food, yeast consumes proteins, peptones, amino acids, as well as ammonium salts. A yeast cell produces many vitamins, and the presence of some growth substances in the medium enhances the growth of yeast. Yeast develops in a relatively wide temperature range (from 3-5 to 38-40 ° C).
In the fermentation processes can participate lower and horse yeast. The latter is used for fermentation occurring at a temperature of 18-30 ° C. Under these conditions, it usually notes a plenty of carbon dioxide and foaming. The yeast themselves rise to the surface of the wandering fluid. High yeast, most often Saccharomyces Cerevisiae, are used in the alcohol industry, bakery, etc., but under some conditions and other yeast consumes.
Low yeast is used for fermentation when reduced temperature (4-10 ° C). At the same time, fermentation is performed calmly, and the mass of yeast cells remains at the bottom of the vessel. Low yeast is often used in the brewing industry, where Saccharomyces Cerevisiae, adapted, however, is usually used, adapted, however, to vital activity at low temperatures. In the winemaking, the yeast of Saccharomyces Vini, S. Cerevisiae Var play an important role. Ellipsoides.
Yeast can grow with a neutral medium reaction, but the fermentation processes are more active at some acidification. Therefore, in practice for breeding yeast, they create a sour Wednesday, which also warns the development of foreign bacterial microflora, poorly carrying low pH.
The value of alcohol fermentation is very large. This process underlies winemaking, brewing, alcohol production, bread accumulation. In these industries, clean yeast cultures are widely used, providing a more correct course of the process and improving product quality.
Yeast and close to them organisms are used to prepare a feed protein. Culturing them on media with a cheap source of carbon nutrition (for example, on molasses, waste of the cellulose or textile industry, methanol, ethanol, etc.), it is possible to obtain a significant mass of yeast containing a full-fledged protein. Yeast is separated and used for feed purposes. Recently, a method for growing feed yeast on the departures of the oil industry has been developed.
Some types of yeast, like other microorganisms, accumulate in their cells a large amount of fat. Similar yeast, which received the technical name "fat", proposed to be used to obtain a microbiological method of fats with valuable technical properties. There are yeast, which accumulate significant amounts of vitamins, based on which they are used in the production of vitamins for medicine and agriculture.
Not all yeast benefit a person, many are capable of calling only carbohydrate oxidation. Among the potent yeast there are pests of food and wines.
Yeast is widespread in nature - in soils, on the surface of plants, etc.