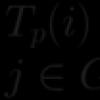What does the carbon react with? Physical and chemical properties of carbon. Qualitative reaction to carbon dioxide
Carbon (C)- typical non-metal; in the periodic system is in the 2nd period of the IV group, the main subgroup. Atomic number 6, Ar = 12.011 amu, nuclear charge +6. Physical properties: carbon forms many allotropic modifications: diamond- one of the hardest substances graphite, coal, soot.
Chemical properties: electronic configuration: 1s2 2 s2 2p2. On the electron shell of an atom - 6 electrons; at the outer valence level - 4 electrons. The most characteristic oxidation states: +4, +2 - in inorganic compounds, - 4, -2 - in organic ones. Carbon in any hybrid state is capable of using all of its valence electrons and orbitals. 4-valent carbon has no lone pairs and no free orbitals - carbon is relatively chemically stable. Several types of hybridization are characteristic: sp, s p2, s p3. At low temperatures, carbon is inert, but when heated, its activity increases. Carbon is a good reducing agent, but it combines with metals and forms carbides, it acts as an oxidizing agent:
Carbon (coke) reacts with metal oxides:


Thus, metal is smelted from ore. At very high temperatures, carbon reacts with many non-metals. It forms a huge amount of organic compounds with hydrogen - hydrocarbons. In the presence of nickel (Ni), carbon, reacting with hydrogen, forms a saturated hydrocarbon - methane: C + H2 = CH4.
When interacting with sulfur, forms carbon disulfide: С + 2S2 = СS2.
At the temperature of the electric arc, carbon combines with nitrogen to form a poisonous gas cyanus: 2C + N2 = C2N2 ?.
In combination with hydrogen, cyanogen forms hydrocyanic acid - HCN. Carbon reacts with halogens depending on their chemical activity, forming halides. In the cold, it reacts with fluorine: C + 2F2 = CF2.
At 2000 ° C in an electric furnace, carbon combines with silicon to form carborundum: Si + C = SiC.
Being in nature: free carbon occurs in the form of diamond and graphite. In the form of compounds, carbon is in the composition of minerals: chalk, marble, limestone - CaCO3, dolomite - MgCO3? CaCO3; hydrocarbonates - Mg (HCO3) 2 and Ca (HCO3) 2, CO2 is part of the air; carbon is the main constituent part of natural organic compounds - gas, oil, coal, peat; it is part of organic substances, proteins, fats, carbohydrates, amino acids that make up living organisms.
MOU "Nikiforovskaya secondary school No. 1"
Carbon and its main inorganic compounds
abstract
Completed: student of grade 9B
Alexander Sidorov
Teacher: Sakharova L.N.
Dmitrievka 2009
Introduction
Chapter I. All About Carbon
1.1. Carbon in nature
1.2. Allotropic modifications of carbon
1.3. Chemical properties of carbon
1.4. Application of carbon
Chapter II. Inorganic carbon compounds
Conclusion
Literature
Introduction
Carbon (Latin Carboneum) C is a chemical element of group IV of Mendeleev's periodic system: atomic number 6, atomic mass 12.011 (1). Consider the structure of the carbon atom. There are four electrons on the outer energy level of the carbon atom. Let's graphically depict:

Carbon has been known since ancient times, and the name of the discoverer of this element is unknown.
At the end of the 17th century. the Florentine scientists Averani and Targioni tried to fuse several small diamonds into one large one and heated them with the help of incendiary glass with the sun's rays. The diamonds disappeared by burning in the air. In 1772, the French chemist A. Lavoisier showed that the combustion of diamond produces CO 2. Only in 1797 the English scientist S. Tennant proved the identity of the nature of graphite and coal. After combustion of equal amounts of coal and diamond, the volumes of carbon monoxide (IV) turned out to be the same.
The variety of carbon compounds, explained by the ability of its atoms to combine with each other and with the atoms of other elements in various ways, determines the special position of carbon among other elements.
Chapter I ... All About Carbon
1.1. Carbon in nature
Carbon is found in nature, both in a free state and in the form of compounds.
Free carbon occurs in the form of diamond, graphite, and carbyne.
Diamonds are very rare. The largest known diamond - "Cullinan" was found in 1905 in South Africa, weighed 621.2 g and measured 10 × 6.5 × 5 cm. The Diamond Fund in Moscow keeps one of the largest and most beautiful diamonds in the world - "Orlov" (37.92 g).
The diamond got its name from the Greek. "Adamas" - invincible, indestructible. The most significant diamond deposits are located in South Africa, Brazil, and Yakutia.
Large deposits of graphite are found in the Federal Republic of Germany, Sri Lanka, Siberia, and Altai.
The main carbon-containing minerals are: magnesite MgCO 3, calcite (limestone, limestone, marble, chalk) CaCO 3, dolomite CaMg (CO 3) 2, etc.
All fossil fuels - oil, gas, peat, bituminous and brown coal, shale - are carbon-based. Some fossil coals are close in composition to carbon, containing up to 99% C.
Carbon accounts for 0.1% of the earth's crust.
In the form of carbon monoxide (IV) CO 2, carbon is part of the atmosphere. A large amount of CO 2 is dissolved in the hydrosphere.
1.2. Allotropic modifications of carbon
Elemental carbon forms three allotropic modifications: diamond, graphite, and carbyne.
1. Diamond is a colorless, transparent crystalline substance that refracts light rays extremely strongly. Carbon atoms in diamond are in the sp 3 -hybridization state. In an excited state, the valence electrons in carbon atoms are unpaired and four unpaired electrons are formed. When chemical bonds are formed, the electron clouds acquire the same elongated shape and are located in space so that their axes are directed to the vertices of the tetrahedron. When the tops of these clouds overlap with the clouds of other carbon atoms, covalent bonds appear at an angle of 109 ° 28 ", and an atomic crystal lattice characteristic of diamond is formed.
Each carbon atom in a diamond is surrounded by four others, located from it in the directions from the center of the tetrahedrons to the vertices. The distance between atoms in tetrahedra is 0.154 nm. The strength of all bonds is the same. Thus, the atoms in a diamond are "packed" very tightly. At 20 ° C, the density of diamond is 3.515 g / cm 3. This explains its exceptional hardness. Diamond does not conduct electric current well.
In 1961, the industrial production of synthetic diamonds from graphite began in the Soviet Union.
In the industrial synthesis of diamonds, pressures of thousands of MPa and temperatures from 1500 to 3000 ° C are used. The process is carried out in the presence of catalysts, which can be some metals, for example Ni. The bulk of the formed diamonds are small crystals and diamond dust.
When heated without air access above 1000 ° C, diamond turns into graphite. At 1750 ° C, the transformation of diamond into graphite occurs rapidly.

Diamond structure
2. Graphite is a gray-black crystalline substance with a metallic luster, greasy to the touch, inferior in hardness even to paper.
Carbon atoms in graphite crystals are in the sp 2 -hybridization state: each of them forms three covalent σ-bonds with neighboring atoms. The angles between the directions of the bonds are equal to 120 °. The result is a grid of regular hexagons. The distance between adjacent nuclei of carbon atoms inside the layer is 0.142 nm. The fourth electron of the outer layer of each carbon atom in graphite is occupied by the p-orbital, which does not participate in hybridization.
Non-hybrid electron clouds of carbon atoms are oriented perpendicular to the plane of the layer and, overlapping with each other, form delocalized σ-bonds. Adjacent layers in a graphite crystal are at a distance of 0.335 nm from each other and are weakly connected to each other, mainly by van der Waals forces. Therefore, graphite has low mechanical strength and is easily split into flakes, which are very strong in themselves. The bond between layers of carbon atoms in graphite is partially metallic in nature. This explains the fact that graphite conducts electric current well, but still not as well as metals.

Graphite structure
The physical properties in graphite differ greatly in directions - perpendicular and parallel to the layers of carbon atoms.
When heated without access to air, graphite does not undergo any changes up to 3700 ° C. At the indicated temperature, it sublimes without melting.
Artificial graphite is obtained from the best grades of coal at 3000 ° C in electric furnaces without access to air.
Graphite is thermodynamically stable over a wide range of temperatures and pressures; therefore, it is accepted as the standard state of carbon. The density of graphite is 2.265 g / cm 3.
3. Carbyne is a fine-crystalline black powder. In its crystal structure, carbon atoms are connected by alternating single and triple bonds in linear chains:
−C≡C − C≡C − C≡C−
This substance was first obtained by V.V. Korshak, A.M. Sladkov, V.I. Kasatochkin, Yu.P. Kudryavtsev in the early 60s of the XX century.
Subsequently, it was shown that carbyne can exist in different forms and contains both polyacetylene and polycumulene chains, in which carbon atoms are linked by double bonds:
C = C = C = C = C = C =
Later, carbyne was found in nature - in meteorite matter.
Carbyne has semiconducting properties; under the influence of light, its conductivity is greatly increased. Due to the existence of different types of bonds and different ways of packing chains of carbon atoms in the crystal lattice, the physical properties of carbyne can vary within wide limits. When heated without air access above 2000 ° C, carbyne is stable; at temperatures around 2300 ° C, its transition to graphite is observed.
Natural carbon is composed of two isotopes
(98.892%) and (1.108%). In addition, insignificant admixtures of a radioactive isotope, which are obtained by artificial means, have been found in the atmosphere.Previously, it was believed that charcoal, soot and coke are close in composition to pure carbon and differ in properties from diamond and graphite, represent an independent allotropic modification of carbon ("amorphous carbon"). However, it was found that these substances are composed of the smallest crystalline particles, in which carbon atoms are linked in the same way as in graphite.
4. Coal - finely ground graphite. Formed by thermal decomposition of carbon-containing compounds without air access. Coals differ significantly in properties depending on the substance from which they are obtained and the method of obtaining. They always contain impurities that affect their properties. The most important types of coal are coke, charcoal, and soot.
Coke is obtained by heating coal without air.
Charcoal is formed when wood is heated without access to air.
Soot is a very fine graphite crystalline powder. Formed during combustion of hydrocarbons (natural gas, acetylene, turpentine, etc.) with limited air access.
Active carbons are porous industrial adsorbents, consisting mainly of carbon. Adsorption is the absorption of gases and dissolved substances by the surface of solids. Activated carbons are obtained from solid fuel (peat, brown and coal, anthracite), wood and products of its processing (charcoal, sawdust, paper waste), tanning industry waste, materials of animal origin, such as bones. Coals with high mechanical strength are produced from coconut shells and other nuts, from fruit seeds. The structure of coals is represented by pores of all sizes; however, the adsorption capacity and the rate of adsorption are determined by the content of micropores in a unit of mass or volume of granules. In the production of active carbon, the starting material is first subjected to heat treatment without access to air, as a result of which moisture and partly tar is removed from it. In this case, a large-pore structure of coal is formed. To obtain a microporous structure, activation is performed either by oxidation with gas or steam, or by treatment with chemical reagents.
Carbon is capable of forming several allotropic modifications. These are diamond (the most inert allotropic modification), graphite, fullerene, and carbyne.
Charcoal and soot are amorphous carbon. Carbon in this state has no ordered structure and actually consists of the smallest fragments of graphite layers. Amorphous carbon treated with hot steam is called activated carbon. 1 gram of activated carbon due to the presence of many pores in it has a total surface of more than three hundred square meters! Due to its ability to absorb various substances, activated carbon is widely used as a filter filler, as well as an enterosorbent for various types of poisoning.
From a chemical point of view, amorphous carbon is its most active form, graphite is moderately active, and diamond is an extremely inert substance. For this reason, the chemical properties of carbon considered below should be primarily attributed to amorphous carbon.
Reducing properties of carbon
As a reducing agent, carbon reacts with non-metals such as oxygen, halogens, sulfur.
Depending on the excess or lack of oxygen when burning coal, carbon monoxide CO or carbon dioxide CO 2 can be formed:
When carbon interacts with fluorine, carbon tetrafluoride is formed:
When carbon is heated with sulfur, carbon disulfide CS 2 is formed:
Carbon is capable of reducing metals after aluminum in a series of activities from their oxides. For instance:
Carbon also reacts with oxides of active metals, but in this case, as a rule, it is not the reduction of the metal that is observed, but the formation of its carbide:
Interaction of carbon with nonmetal oxides
Carbon enters into a co-proportionation reaction with carbon dioxide CO 2:
One of the most important industrial processes is the so-called steam conversion of coal... The process is carried out by passing water vapor through hot coal. In this case, the following reaction occurs:
At high temperatures, carbon is capable of reducing even such an inert compound as silicon dioxide. In this case, depending on the condition, the formation of silicon or silicon carbide ( carborundum):
Also, carbon as a reducing agent reacts with oxidizing acids, in particular, concentrated sulfuric and nitric acids:
Oxidizing properties of carbon
The chemical element carbon is not highly electronegative; therefore, the simple substances it forms rarely exhibit oxidizing properties in relation to other non-metals.
An example of such reactions is the interaction of amorphous carbon with hydrogen when heated in the presence of a catalyst:
and also with silicon at a temperature of 1200-1300 ° C:
Carbon exhibits oxidizing properties in relation to metals. Carbon is capable of reacting with active metals and some metals of average activity. Reactions take place when heated:
| Carbides of active metals are hydrolyzed by water:
as well as solutions of non-oxidizing acids: This results in the formation of hydrocarbons containing carbon in the same oxidation state as in the original carbide. |
Silicon chemical properties
Silicon can exist, like carbon, in a crystalline and amorphous state, and, as in the case of carbon, amorphous silicon is significantly more chemically active than crystalline.
Sometimes amorphous and crystalline silicon is called allotropic modifications, which, strictly speaking, is not entirely true. Amorphous silicon is essentially a conglomerate of the smallest particles of crystalline silicon randomly arranged relative to each other.
Interaction of silicon with simple substances
non-metals
Under normal conditions, silicon, due to its inertness, reacts only with fluorine:
Silicon reacts with chlorine, bromine and iodine only when heated. In this case, it is characteristic that, depending on the activity of the halogen, a correspondingly different temperature is required:
So with chlorine, the reaction proceeds at 340-420 ° C:
With bromine - 620-700 o C:
With iodine - 750-810 o C:
The reaction of silicon with oxygen proceeds, however, it requires very strong heating (1200-1300 ° C) due to the fact that a strong oxide film makes it difficult to interact:
At a temperature of 1200-1500 ° C, silicon slowly interacts with carbon in the form of graphite to form silicon carbide SiC - a substance with an atomic crystal lattice similar to diamond and almost as strong as it:
Silicon does not react with hydrogen.
metals
Due to its low electronegativity, silicon can exhibit oxidizing properties only in relation to metals. Of the metals, silicon reacts with active (alkaline and alkaline-earth) metals, as well as with many metals of medium activity. As a result of this interaction, silicides are formed:
Interaction of silicon with complex substances
Silicon does not react with water even when boiling, however, amorphous silicon interacts with superheated water vapor at a temperature of about 400-500 o C. In this case, hydrogen and silicon dioxide are formed:
Of all acids, silicon (in an amorphous state) reacts only with concentrated hydrofluoric acid:
Silicon dissolves in concentrated alkali solutions. The reaction is accompanied by the evolution of hydrogen.
Free carbon is a typical reducing agent. When oxidized with oxygen in excess air, it turns into carbon monoxide (IV):
with a lack of - into carbon monoxide (II):
Both reactions are highly exothermic.
When carbon is heated in an atmosphere of carbon monoxide (IV), carbon monoxide is formed:
Carbon reduces many metals from their oxides:
This is how reactions proceed with oxides of cadmium, copper, lead. When carbon interacts with oxides of alkaline earth metals, aluminum and some other metals, carbides are formed:

This is explained by the fact that active metals are stronger reducing agents than carbon, therefore, when heated, the resulting metals are oxidized with an excess of carbon:

Carbon monoxide (II).
Incomplete oxidation of carbon produces carbon monoxide (II) CO - carbon monoxide. It is poorly soluble in water. The formal oxidation state of carbon 2+ does not reflect the structure of the CO molecule.
In the CO molecule, in addition to the double bond formed by the sharing of electrons of carbon and oxygen, there is an additional, third bond (shown by an arrow), formed by the donor-acceptor mechanism due to the lone pair of oxygen electrons
In this regard, the CO molecule is extremely strong. Carbon monoxide (II) is non-salt-forming and does not interact under normal conditions with water, acids and alkalis. At elevated temperatures, it is prone to addition and oxidation-reduction reactions. In air, CO burns with a blue flame:
It recovers metals from their oxides:
![]()
Under the influence of radiation in direct sunlight or in the presence of catalysts, CO combines with the formation of phosgene, an extremely poisonous gas:
With many metals, CO forms volatile carbonyls:
A covalent bond in a nickel carbonyl molecule is formed by a donor-acceptor mechanism, with the electron density shifting from a carbon atom to a nickel atom. The increase in the negative charge on the metal atom is compensated by the participation of its d-electrons in the bond, so the oxidation state of the metal is 0. When heated, metal carbonyls decompose into metal and carbon monoxide (II), which is used to obtain metals of special purity.
In nature, carbon monoxide (II) is practically not found. It can be formed during dehydration of formic acid (laboratory method of obtaining):
Based on the last transformation, purely formally, CO can be considered an anhydride of formic acid. This is confirmed by the following reaction, which occurs when CO is passed into the alkali melt at high pressure:
Carbon monoxide (IV) and carbonic acid. Carbon monoxide (IV) is an anhydride of carbonic acid and has all the properties of acid oxides (see § 8).
When dissolved in water, carbonic acid is partially formed, while the following equilibrium exists in the solution.
CHEMICAL PROPERTIES OF CARBON
Carbon is inactive, in the cold it reacts only with fluorine; chemical activity manifests itself at high temperatures.
Memo! "Chemical properties"
|
C - reducing agent С 0 - 4 е - → С +4 or С 0 - 2 е - → С +2 |
C - oxidizing agent C 0 + 4 e - → C -4 |
|
1) with oxygen C 0 + O 2 t ˚ C → CO 2 carbon dioxide Experience with a lack of oxygen, incomplete combustion is observed, carbon monoxide is formed: 2C 0 + O 2 t ˚ C → 2C +2 O 2) with fluorine C + 2F 2 → CF 4 3) with water vapor C 0 + H 2 O t ˚ C → С +2 O + H 2 water gas 4) with metal oxides C +Me x O y = CO 2 + Me C 0 + 2CuO t˚C → 2Cu + C +4 O 2 5) with acids - oxidizing agents: C 0 + 2 H 2 SO 4 (conc.) → C +4 O 2 + 2 SO 2 + 2 H 2 O С 0 + 4 HNO 3 (conc.) → С +4 O 2 + 4 NO 2 + 2 H 2 O |
1) forms carbides with some metals 4 Al + 3 C 0 t ˚ C → Al 4 C 3 -4 Ca + 2 C 0 t ˚ C → CaC 2 -1 2) with hydrogen C 0 + 2H 2 t˚C → CH 4 |
Adsorption
The reverse process is the release of these absorbed substances - desorption.
Adsorption application
Purification from impurities (in the production of sugar, etc.), for the protection of the respiratory system (gas masks), in medicine (tablets "Karbolen"), etc.
Application of carbon
Diamonds are widely used for cutting rocks and grinding extremely hard materials. Diamonds are cut into jewelry. Graphite is used to make inert electrodes and pencil leads. Mixed with industrial oils as a lubricant. Melting crucibles are made from a mixture of graphite and clay. Graphite is used in the nuclear industry as a neutron absorber.
Coke is used in metallurgy as a reducing agent. Charcoal - in forges, to obtain gunpowder (75% KNO 3 + 13% C + 12% S), to absorb gases (adsorption), as well as in everyday life. Soot is used as a rubber filler, for the manufacture of black paints - printing ink and ink, as well as in dry electrochemical cells. Glassy carbon is used for the manufacture of equipment for highly corrosive environments, as well as in aviation and astronautics.
Activated carbon absorbs harmful substances from gases and liquids: it is filled with gas masks, purification systems, it is used in medicine for poisoning.
CHARCOAL
Charcoal- a microporous high-carbon product formed during decomposition of wood without air access. It is used in the production of crystalline silicon, carbon disulfide, ferrous and non-ferrous metals, activated carbon, etc., and also as a household fuel (specific heat of combustion 31.5-34 MJ / kg).


TASKS FOR ANCHORING
# 1. Complete the reaction equations, make an electronic balance, indicate the oxidizing agent and the reducing agent for each reaction:
C + O 2 (h) =
C + O 2 (insufficient) =
C + H 2 =
C + Ca =
C + Al =