Chemistry tutor manual. Salts of oxygen acids of chlorine Oxygen-containing perchloric acids
PHYSICOCHEMICAL CHARACTERISTICS
Chlorine forms a number of oxygen acids - hypochlorous HCIO, chlorous HCIO2, hypochlorous HCIO3 and perchloric HC in the equation for the dependence of the concentration of chlorine dioxide in solution With(V mol/l) from its partial pressure P (in mmHg Art.) with =KR at 0, 5, 10, 25 and 35°, respectively, are: 70.6, 56.3, 46.2, 30.2 and 21.5. With increasing temperature, the solubility of chlorine dioxide in water decreases sharply. The solubility of CSO in other solvents (CC14, H2SO4 and CH3COOH) also obeys Henry’s Law34. In aqueous solutions in the cold, chlorine dioxide decomposes extremely slowly; in hot water it decomposes with the formation of HCIO3, CI2 and O2. The existence of crystalline hydrate C102 6H2035 has been established.
It is assumed that chlorine dioxide is an anhydride36 that forms with water the corresponding acids H2CIO3 and H2CI2O5, which are very unstable and can be reduced by metals to HCl2. In the absence of reducing agents, the rate of decomposition of these acids is higher than the rate of their formation. Chlorine dioxide reacts with hydrogen peroxide to form chlorous acid37: 2СУ2 + Н202 = 2НС102 + 02
Chlorine dioxide irritates the respiratory tract and causes headaches even at a dilution of 45: 1,000.
Chlorous acid 38-40 is also isolated in free form, but is usually obtained in aqueous solutions. Its dissociation constant is 1.07-10-2 at 18°. The formation of chlorous acid occurs in significant quantities only in a strongly acidic environment (pH<3). При этом в растворе наряду с хлористой кислотой находится и двуокись хлора 4I.
Chlorites - salts of chlorous acid in the solid state under ordinary conditions are quite stable compounds. Acidic aqueous solutions decompose the faster the higher the temperature and the lower the pH value. Alkaline solutions are quite stable42. Some chlorites can be prepared by the action of free chlorous acid on insoluble carbonates43. Sodium chlorite crystallizes from an alkaline solution in the form of anhydrous salt NaC102 and trihydrate NaC102-3H20, which turns into anhydrous salt at 37.4°44. When heated to 175°, it decomposes with the release of oxygen. The reaction proceeds at high speed until it explodes. In slightly alkaline solutions containing no more than 1 g-mol/l NaC102, sodium chlorite does not decompose when boiled. In more concentrated solutions it decomposes according to reactions 45,46:
3 NaCl02 = 2 NaClC>3 + NaClNaC102 - NaCl+ 02
The rate constants of these reactions are equal47 respectively at 103°: 0.65-10-6 and 1.2-10"7; at 83°: 1.6-10-7 and 0.2-10"8.
Hypochlorous acid can only exist in free form in solution. It is a strong acid and a vigorous oxidizing agent. Its salts - chlorates - are mostly highly soluble in water; in solutions they are not oxidizing agents.
Potassium chlorate or Bertholet's salt KSYuz crystallizes in anhydrous form in the form of transparent colorless crystals of a monoclinic system with a density of 2.32 g/cm3. Solubility of KS103 in water: at 0° - 3.21%, at 104° (boiling point) - 37.6%. When heated to 368.4°, KSUS melts and then begins to decompose according to the reactions:
2KSYuz = 2KS1 +302 +23.6 to feces 4KS103 = ZKSYu4 + KS1 + 70.9 kcal
The resulting products (KS1 and KS104) accelerate48 the release of oxygen. At 610°, the resulting potassium perchlorate melts and decomposes:
KSYu4 = KS1 + 202 - 7.9kcal
In the presence of catalysts (MnO2 and others), potassium chlorate decomposes at lower temperatures with intense release of oxygen. Potassium chlorate in an acidic environment is a strong oxidizing agent. Mixtures of it with coal, sulfur and other substances explode on impact. Potassium chlorate (and other chlorates) is poisonous (lethal dose - 2-3g KSO3).
Sodium chlorate NaC103 crystallizes in anhydrous form, is highly hygroscopic, and diffuses in air. A saturated aqueous solution contains 41.9% at -15° and 74.1% NaC103 at 122°. The melting point of sodium chlorate is in the range of 248-264°. There have been cases of explosions of sodium chlorate in warehouses during storage, as well as inflammation of dry parts of plants that were exposed to sodium chlorate. In the presence of hygroscopic substances (CaCl2, MgCl2, etc.) 4E, as well as polyborates or sodium metaborates, the explosive and flammable hazard of sodium chlorate is reduced. In the NaC103-NaC102-H20 50 system, anhydrous NaC103 and NaCl02, as well as NaC102-3H20, crystallize in the temperature range 15-45°.
Calcium chlorate Ca(Cl3)2 crystallizes from an aqueous solution in the form of a dihydrate51, melting at 130°. A saturated aqueous solution boils at 182°. Anhydrous calcium chlorate decomposes when heated to 334°.
Magnesium chlorate hexahydrate Mg(C103)2 6H20 is orthorhombic crystals - long needles or leaves. At 35° it partially melts and turns into tetrahydrate. Its solubility in water is 53% at 0°, 56.5% at 18°, 60.23% at 29° and 63.65% at 35°. It is highly hygroscopic, does not explode and is fire safe49.
Perchloric acid52 forms two crystalline hydrates - HC104 4H20 and HCIO4 3H20 53 and is a strong electrolyte54. The activity coefficient of perchloric acid at 25° changes from 0.911 to 0.804 when the concentration of HCIO4 changes from 0.01 to 0.1 M in 1 kg solution®5.
Potassium perchlorate KSIu4 forms orthorhombic crystals with a density of 2.52 g/cm3. At 0 to 100 ml water dissolves 0.75 G, and at 100° - 21.8 g KSO4. Pure potassium perchlorate decomposes at 537-600° into KC1 and 02. KC103 is formed as an intermediate product, which, when melted, accelerates decomposition56. The reaction is accelerated in the presence of KCl, KBr, KI57, Cu, Fe, Co, MgO, etc.58.
Magnesium perchlorate forms crystalline hydrates with 2, 4 and 6 water molecules. The equilibrium vapor pressure at 23° above Mg(C104)2 6H20 is 20.9 mmHg Art., over Mg(C104)2 4H20- 8.15 mmHg Art., and above Mg(C104)2-2H20 about Yu-4-Sh-5 mm rt. Art. 5E. When heated above 400°, Mg(C104)2 decomposes60.
Ammonium perchlorate is characterized by the highest oxygen content by weight among all perchlorates. 10.7 dissolves in 100 g at 0° G, at 85° - 42.5 G NH4CIO4. In a mutual water system of perchlorates and chlorides. ammonium and magnesium, the least soluble salt at 25° is NH4CIO461.
Oxygen compounds of chlorine of higher oxidation states are fire and explosive, especially in the presence of easily oxidized impurities, such as organic substances, from which they should be protected from contamination. Explosion of solid dry chlorates and perchlorates can be caused by impact or strong shock, which must be taken into account when drying, grinding and transporting these in< ществ. Эти операции должны осуществляться в аппаратах, в которых исключена возможность ударов металлических частей.
APPLICATION
Salts of lower oxygen acids of chlorine are good bleaching agents due to their high oxidative activity. The main bleaching and oxidizing chlorine compound is bleach62. Currently, hypochlorites, chlorites and chlorine dioxide are also widely used for these purposes.
The largest quantities of bleach are used in the textile and paper industries to bleach fabrics and pulp (bleach is often called bleaching lime). Chloride of lime is used as an oxidizing agent in some chemical industries (in the production of chloroform, chloropicrin and other products), for the disinfection of drinking and waste water, for the disinfection of vegetable stores63 and as a good degasser. It is also used to purify acetylene and some petroleum products.
Chloride of lime is produced in three grades (Table 112).
Losses of active chlorine in grade A bleach should be no more than 4% during 3 years of its storage from the date of shipment by the plant.
Chloride of lime, grades B and C, is packaged in wooden barrels with a capacity of 50 to 275 l, into plywood stamped barrels or plywood drums with a capacity of 50 and 100 l, and also (for short-term storage) in dry, filled wooden barrels with a capacity of 50 to 250 l. Chloride of lime, grade A, as well as grade B (for long-term storage) is packaged in steel drums with a capacity of 100 l. Barrels or drums with bleach are hermetically sealed and stored in a dry and cool room, protected from direct sunlight. Instead of wooden barrels and drums, plastic bags are also used.
Despite these precautions, bleach gradually loses active chlorine during storage. If the container is not tightly sealed, some product samples almost completely lose active chlorine within one year, and sometimes much sooner. At 40-45°, ordinary bleach completely loses its activity within 2 months.
Chloride of lime is increasingly being replaced by other more convenient bleaching and oxidizing substances62 - hypochlorites, chlorine dioxide, etc.
Sodium hypochlorite in the form of an aqueous solution is widely used due to the ease of its production at the point of consumption. It is an intermediate product 64 in the production of hydrazine, plastics, synthetic fibers, etc. A hypochlorite method has been proposed 65 for processing dust-like waste from sharpening carbide tools, based on the oxidation of tungsten carbide in alkaline solutions of NaCIO and the transition of tungsten into solution.
According to GOST 11086-64, sodium hypochlorite must be a transparent greenish-yellow liquid without sediment or suspended particles, containing at least 185 g/l active chlorine and not more than 0.07 g/l gland; NaOH content should be within 10-20 g/l. Sodium hypochlorite solution is stored and transported in closed gummed or vinyl-protected tanks and containers at a temperature not exceeding 25°.
Technical calcium hypochlorite, containing more than 50% active chlorine, is more transportable than bleach. Less than 100% of ballast (impurities and containers) is transported with calcium hypochlorite, while 250-300% is transported with bleach. An important advantage of calcium hypochlorite, compared to bleach, is the absence of significant sediment when dissolving it in water66 (when dissolving bleach, a precipitate of basic salts is formed, in which up to 50% of active chlorine is sometimes lost). It was proposed67 to use a mixture of 2 wt. parts Ca(OS1)2 and 0.8 wt. including Na2S04 in the form of tablets for water treatment.
Calcium hypochlorite is produced in the form of a dibasic salt 3Ca(CiO)2 2Ca (OH)2 2H20, designated DTSGK, and less often in the form of dibasic calcium hypochlorite Ca (C10)2 2Ca(OH)2, designated DSGK - GOST 13392-67 provides for the release of DTSGK
and 2nd grade. They must contain, respectively: active chlorine not less than 55 and 50% and moisture not more than 1 and 1.5%; the total chlorine content should not exceed half the active chlorine content (%) plus 6% for 1st grade, or plus 7% for
DTSGK is packed in galvanized drums. The product must be stored in a dry, unheated room.
Chlorine dioxide, in its oxidizing properties, occupies an intermediate position between chlorates and hypochlorites. Its main advantage as a bleaching reagent is that it has almost no destructive effect on the fiber of the fibers. Therefore it is widely used How The best bleaching agent for wood (paper) pulp and cellulose, as well as for the sterilization and deodorization of water68 and food products. Due to the difficulty of storage and transportation, COG is usually obtained at the point of consumption and used as a 10% mixture with air69.
Sodium chlorite is widely used in the textile industry for bleaching fabrics, yarn, and fiber. In this case, high quality bleaching is achieved without reducing the strength of the fiber. It is also used as a starting material for the production of small quantities of chlorine dioxide.
Potassium chlorate is used mainly in the match industry, in pyrotechnics, in small quantities in the pharmaceutical industry, and also in explosives.
The composition of technical potassium chloride salt must correspond to the data in Table. 113.
TABLE 113
Composition of technical bertoletasalt(ByGOST 2713-70)
Potassium chlorate (in terms of dry matter), not Nope.....
Moisture, no more................................................... ...................................
Not soluble V substances in water, not more................................
Chlorides (in terms of CaC12), no more...................................
Sulfates (in terms of CaS04), no more...................................
Bromates (in terms of KVg03), no more...................................
Alkali (in terms of CaO), no more......................................
Organic substances, no more................................................... ....
Heavy metals (in terms of Pb), no more. . . . Iron (Fe), not salt
Sodium chlorate is used as a herbicide and defoliant (in limited quantities due to its hygroscopicity). It is mainly used as an intermediate for the production of other chlorates, potassium perchlorate, perchloric acid, chlorine dioxide and sodium chlorite. Some (small) amounts of sodium chlorate are used to bleach cellulose. The use of NaC103 for the manufacture of candles, which are a source of oxygen on nuclear submarines, has been described70.
The composition of technical sodium chlorate, crystalline and solution (or pulp), according to GOST 12257-66, must meet the requirements given in table. 114.
TABLE U4
Composition of technical sodium chlorate (GOST 12257-66)
|
0,7* 0,3* 0,2* |
* In terms of 100% product.
Berthollet salt and sodium chlorate are packaged in bags of polyethylene or polyvinyl chloride film, enclosed galvanized steel drums or coated with perchlorovinyl varnish, or in bags made of chlorine fabric (also with a film liner).
Calcium chlorate is a general herbicide and is widely used to kill weeds.
Magnesium chlorate also serves as a herbicide and, in addition, is a defoliant used for pre-harvest removal of cotton leaves 71>72, and in large doses it can serve as a desiccant for pre-harvest drying of cotton and other plants.
Magnesium chlorate (defoliant), according to GOST 10483-66, must contain 60 ± 2% Mg(C103)2 6H20 and no more than 0.6% water-insoluble residue; the temperature at which it begins to melt should not be lower than 44°. It is transported in sealed drums made of black roofing steel or in paper bitumen-coated five-layer bags with a liner made of polyethylene or polyvinyl chloride film.
Perchlorates are used in the production of explosives and pyrotechnic materials52-73. Mixtures containing ~60% KS104, which form hygroscopic smoke to regulate atmospheric precipitation, have been proposed74.
Among perchlorates, ammonium perchlorate is of particular importance, used for the manufacture of smokeless explosives75-76. Heavy metal perchlorates and perchloric acid are used as electrolytes in electroplating, carburization, etc. In the presence of HC104, dense, shiny palladium deposits are obtained on electrolytically polished copper77. Indicate78 on the possibility of rhenium re-extraction with perchloric acid from organic solvents.
Chlorine- element of the 3rd period and VII A-group of the Periodic Table, serial number 17. Electronic formula of the atom [ 10 Ne ]3s 2 Зр 5, characteristic oxidation states 0, -1, + 1, +5 and +7. The most stable state is Cl -1. Chlorine oxidation state scale:
7 – Cl 2 O 7 , ClO 4 – , HClO 4 , KClO 4
5 - ClO 3 - , HClO 3 , KClO 3
1 – Cl 2 O, ClO -, HClO, NaClO, Ca(ClO) 2
- 1 - Cl - , HCl, KCl, PCl 5
Chlorine has a high electronegativity (2.83) and exhibits non-metallic properties. It is part of many substances - oxides, acids, salts, binary compounds.
In nature - twelfth element by chemical abundance (fifth among non-metals). It is found only in a chemically bound form. The third most abundant element in natural waters (after O and H), there is especially a lot of chlorine in sea water (up to 2% by weight). A vital element for all organisms.
Chlorine C1 2. Simple substance. Yellow-green gas with a pungent suffocating odor. The Cl 2 molecule is nonpolar and contains a C1-C1 σ bond. Thermally stable, non-flammable in air; a mixture with hydrogen explodes in light (hydrogen burns in chlorine):
Cl 2 +H 2 ⇌HCl
It is highly soluble in water, undergoes 50% dismutation in it and completely in an alkaline solution:
Cl 2 0 +H 2 O ⇌HCl I O+HCl -I
Cl 2 +2NaOH (cold) = NaClO+NaCl+H 2 O
3Cl 2 +6NaOH (hor) =NaClO 3 +5NaCl+H 2 O
A solution of chlorine in water is called chlorine water, in the light, the acid HClO decomposes into HCl and atomic oxygen O 0, so “chlorine water” must be stored in a dark bottle. The presence of acid HClO in “chlorine water” and the formation of atomic oxygen explain its strong oxidizing properties: for example, many dyes become discolored in wet chlorine.
Chlorine is a very strong oxidizing agent towards metals and non-metals:
Сl 2 + 2Nа = 2NаСl 2
ЗСl 2 + 2Fe→2FeСl 3 (200 °C)
Сl 2 +Se=SeCl 4
Cl 2 + Pb → PbCl 2 (300°WITH)
5Cl 2 +2P→2PCl 5 (90 °C)
2Cl 2 +Si→SiCl 4 (340 °C)
Reactions with compounds of other halogens:
a) Cl 2 + 2KVg (P) = 2KCl + Br 2 (boiling)
b) Сl 2 (week) + 2КI (р) = 2Кl + I 2 ↓
3Cl (ex.) + 3H 2 O+ KI = 6HCl + KIO 3 (80 °C)
Qualitative reaction- interaction of CL 2 deficiency with KI (see above) and detection of iodine by blue color after adding starch solution.
Receipt chlorine in industry:
2NаСl (melt) → 2Nа + Сl 2 (electrolysis)
2NaCl+ 2H 2 O→H 2 + Cl 2+ 2NaOH (electrolysis)
and in laboratories:
4HCl (conc.) + MnO 2 = Cl 2 + MnCl 2 + 2H 2 O
(similarly with the participation of other oxidizing agents; for more details, see reactions for HCl and NaCl).
Chlorine is a product of basic chemical production and is used to produce bromine and iodine, chlorides and oxygen-containing derivatives, to bleach paper, and as a disinfectant for drinking water. Poisonous.
Hydrogen chloride NS l . Anoxic acid. A colorless gas with a pungent odor, heavier than air. The molecule contains a covalent σ bond H - Cl. Thermally stable. Very soluble in water; dilute solutions are called hydrochloric acid, and the smoking concentrated solution (35-38%) - hydrochloric acid(the name was given by alchemists). Strong acid in solution, neutralized by alkalis and ammonia hydrate. A strong reducing agent in a concentrated solution (due to Cl - I), a weak oxidizing agent in a dilute solution (due to H I). An integral part of “royal vodka”.
The qualitative reaction to the Cl ion is the formation of white precipitates AgCl and Hg 2 Cl 2, which are not transferred into solution by the action of dilute nitric acid.
Hydrogen chloride serves as a raw material in the production of chlorides, organochlorine products, and is used (in the form of a solution) in the etching of metals and the decomposition of minerals and ores. Equations of the most important reactions:
HCl (dil.) + NaOH (dil.) = NaCl + H 2 O
HCl (dil.) + NH 3 H 2 O = NH 4 Cl + H 2 O
4HCl (conc., horizontal) + MO 2 = MCl 2 + Cl 2 + 2H 2 O (M = Mn, Pb)
16HCl (conc., horizontal) + 2KMnO 4 (s) = 2MnCl 2 + 5Cl 2 + 8H 2 O + 2KCl
14HCl (conc.) + K 2 Cr 2 O 7 (t) = 2СrСl 3 + 3Сl 2 + 7Н 2 O + 2КCl
6HCl (conc.) + KClO 3(T) = KCl + 3Cl 2 + 3H 2 O (50-80 °C)
4HCl (conc.) + Ca(ClO) 2(t) = CaCl 2 + 2Cl 2 + 2H 2 O
2HCl (dil.) + M = MCl 2 + H 2 (M = Re, 2p)
2HCl (dil.) + MSO 3 = MCl 2 + CO 2 + H 2 O (M = Sa, Va)
HCl (dil.) + AgNO 3 = HNO 3 + AgCl↓
The production of HCl in industry is the combustion of H 2 into Cl 2 (see), in the laboratory - displacement from chlorides with sulfuric acid:
NaCl (t) + H 2 SO4 (conc.) = NaHSO 4 + NSl(50 °C)
2NaCl (t) + H 2 SO 4 (conc.) = Na 2 SO 4 + 2HCl(120 °C)
Chlorides
Sodium chloride Na Cl . Oxygen-free salt. Common name salt. White, slightly hygroscopic. Melts and boils without decomposition. Moderately soluble in water, solubility depends little on temperature, the solution has a characteristic salty taste. Does not undergo hydrolysis. Weak reducing agent. Enters into ion exchange reactions. Subject to electrolysis in melt and solution.
It is used to produce hydrogen, sodium and chlorine, soda, caustic soda and hydrogen chloride, as a component of cooling mixtures, a food product and a preservative.
In nature, the bulk of rock salt deposits, or halite, And sylvinite(together with KCl), brine of salt lakes, mineral impurities of sea water (NaCl content = 2.7%). In industry it is obtained by evaporation of natural brines.
Equations of the most important reactions:
2NaCl (s) + 2H 2 SO 4 (conc.) + MnO 2 (s) = Cl 2 + MnSO 4 + 2H 2 O + Na 2 SO 4 (100 °C)
10NаСl (t) + 8Н 2 SO 4 (conc.) + 2КМnO 4 (t) = 5Сl 2 + 2МnSO 4 + 8Н 2 О + 5Nа 2 SO 4 + К 2 SO 4 (100°C)
6NaCl (T) + 7H 2 SO 4 (conc.) + K 2 Cr 2 O 7 (t) = 3Cl 2 + Cr 2 (SO 4) 3 + 7H 2 O+ ZNa 2 SO 4 + K 2 SO 4 (100 °C)
2NaCl (s) + 4H 2 SO 4 (conc.) + PbO 2 (s) = Cl 2 + Pb(HSO 4) 2 + 2H 2 O + 2NaHSO 4 (50 °C)
NaСl (diluted) + AgNO 3 = NaNO 3 + AgСl↓
NaCl (l) →2Na+Cl 2 (850°С, electrolysis)
2NaCl + 2H 2 O→H 2 + Cl 2 + 2NaOH (electrolysis)
2NаСl (р,20%) → Сl 2 + 2 Na(Ng) "amalgam"(electrolysis, onHg-cathode)
Potassium chloride KCl . Oxygen-free salt. White, non-hygroscopic. Melts and boils without decomposition. Moderately soluble in water, the solution has a bitter taste, there is no hydrolysis. Enters into ion exchange reactions. It is used as a potassium fertilizer to produce K, KOH and Cl 2. In nature, the main component (along with NaCl) of deposits is sylvinite.
The equations for the most important reactions are the same as those for NaCl.
Calcium chloride CaCl 2 . Oxygen-free salt. White, melts without decomposition. Dissolves in air due to vigorous absorption of moisture. Forms crystalline hydrate CaCl 2 6H 2 O with a dehydration temperature of 260 °C. Highly soluble in water, no hydrolysis. Enters into ion exchange reactions. Used for drying gases and liquids and preparing cooling mixtures. A component of natural waters, an integral part of their “permanent” hardness.
Equations of the most important reactions:
CaCl 2(T) + 2H 2 SO 4 (conc.) = Ca(HSO 4) 2 + 2HCl (50 °C)
CaCl 2(T) + H 2 SO 4 (conc.) = CaSO 4 ↓+ 2HCl (100 °C)
CaCl 2 + 2NaOH (conc.) = Ca(OH) 2 ↓+ 2NaCl
ZCaCl 2 + 2Na 3 PO 4 = Ca 3 (PO 4) 2 ↓ + 6NaCl
CaCl 2 + K 2 CO 3 = CaCO 3 ↓ + 2КCl
CaCl 2 + 2NaF = CaF 2 ↓+ 2NaCl
CaCl 2(l) → Ca + Cl 2 (electrolysis,800°С)
Receipt:
CaCO 3 + 2HCl = CaCl 2 + CO 3 + H 2 O
Aluminum chloride AlCl 3 . Oxygen-free salt. White, fusible, highly volatile. The pair consists of covalent monomers AlCl 3 (triangular structure, sp 2 hybridization, predominate at 440-800 ° C) and dimers Al 2 Cl 6 (more precisely, Cl 2 AlCl 2 AlCl 2, structure - two tetrahedra with a common edge, sp 3 -hybridization, predominate at 183-440 °C). It is hygroscopic and “smoke” in the air. Forms a crystalline hydrate that decomposes when heated. It is highly soluble in water (with a strong exo-effect), completely dissociates into ions, and creates a strongly acidic environment in solution due to hydrolysis. Reacts with alkalis, ammonia hydrate. Recovered by electrolysis of the melt. Enters into ion exchange reactions.
Qualitative reaction on the Al 3+ ion - the formation of an AlPO 4 precipitate, which is transferred into solution with concentrated sulfuric acid.
It is used as a raw material in the production of aluminum, a catalyst in organic synthesis and oil cracking, and a chlorine carrier in organic reactions. Equations of the most important reactions:
AlCl 3. 6H 2 O →AlCl(OH) 2 (100-200°С, —HCl, H 2 O) →Al 2 O 3 (250-450°С,-HCl,H2O)
AlCl 3(t) + 2H 2 O (moisture) = AlCl(OH) 2(t) + 2HCl (White smoke")
AlCl 3 + 3NaON (diluted) = Al(OH) 3 (amorphous) ↓ + 3NaCl
AlCl 3 + 4NaOH (conc.) = Na[Al(OH) 4 ] + 3NaCl
AlCl 3 + 3(NH 3 . H 2 O) (conc.) = Al(OH) 3 (amorphous) + 3NH 4 Cl
AlCl 3 + 3(NH 3 H 2 O) (conc.) = Al (OH) ↓ + ZNH 4 Cl + H 2 O (100°C)
2Al 3+ + 3H 2 O + 3SO 2- 3 = 2Al(OH) 3 ↓ + 3CO 2 (80°C)
2Al 3+ =6H 2 O+ 3S 2- = 2Al(OH) 3 ↓+ 3H 2 S
Al 3+ + 2HPO 4 2- — AlPO 4 ↓ + H 2 PO 4 —
2АlСl 3 →2Аl + 3Сl 2 (electrolysis, 800 °C ,in the meltNаСl)
Receipt AlCl in industry and - chlorination of kaolin, alumina or bauxite in the presence of coke:
Al 2 O 3 + 3C (coke) + 3Cl 2 = 2AlCl 3 + 3CO (900 °C)
Ferric chloride( II ) F EU l 2 . Oxygen-free salt. White (hydrate bluish-green), hygroscopic. Melts and boils without decomposition. When heated strongly, it is volatile in a flow of HCl. Fe-Cl bonds are predominantly covalent, the pair consists of FeCl 2 monomers (linear structure, sp-hybridization) and Fe 2 Cl 4 dimers. Sensitive to oxygen in the air (darkens). It is highly soluble in water (with a strong exo-effect), completely dissociates into ions, and weakly hydrolyzes at the cation. When the solution is boiled, it decomposes. Reacts with acids, alkalis, ammonia hydrate. Typical reducer. Enters into ion exchange and complexation reactions.
It is used for the synthesis of FeCl and Fe 2 O 3, as a catalyst in organic synthesis, a component of drugs against anemia.
Equations of the most important reactions:
FeCl 2 4H 2 O = FeCl 2 + 4H 2 O (220 °C, atm.N 2 )
FeCl 2 (conc.) + H 2 O=FeCl (OH)↓ + HCl (boiling)
FeCl 2 (t) + H 2 SO 4 (conc.) = FeSO 4 + 2HCl (boiling)
FeCl 2(t) + 4HNO 3 (conc.) = Fe(NO 3) 3 + NO 2 + 2HCl + H 2 O
FeCl 2 + 2NaOH (dil.) = Fe(OH) 2 ↓+ 2NaCl (in atm.N 2 )
FeCl 2 + 2(NH 3 . H 2 O) (conc.) = Fe(OH) 2 ↓ + 2NH 4 Cl (80 °C)
FeCl 2 + H 2 = 2HCl + Fe (extra pure, above 500 °C)
4FeCl 2 + O 2 (air) → 2Fe(Cl)O + 2FeCl 3 (t)
2FeCl 2(p) + Cl 2 (ex.) = 2FeCl 3(p)
5Fe 2+ + 8H + + MnO - 4 = 5Fe 3+ + Mn 2+ + 4H 2 O
6Fe 2+ + 14Н + + Сr 2 O 7 2- = 6Fe 3+ + 2Сr 3+ +7Н 2 O
Fe 2+ + S 2- (divided) = FeS↓
2Fe 2+ + H 2 O + 2CO 3 2- (diluted) = Fe 2 CO 3 (OH) 2 ↓+ CO 2
FeСl 2 →Fe↓ + Сl 2 (90°C, diluted with HCl, electrolysis)
Receive e: interaction of Fe with hydrochloric acid:
Fe + 2HCl = FeCl 2+ H 2
(V industry Hydrogen chloride is used and the process is carried out at 500 °C).
Ferric chloride( III ) F EU l 3 . Oxygen-free salt. Black-brown (dark red in transmitted light, green in reflected light), the hydrate is dark yellow. When melted it turns into a red liquid. Very volatile, decomposes when heated strongly. Fe-Cl bonds are predominantly covalent. The steam consists of FeCl 3 monomers (triangular structure, sp 2 -hybridization, predominate above 750 °C) and Fe 2 Cl 6 dimers (more precisely, Cl 2 FeCl 2 FeCl 2, structure - two tetrahedra with a common edge, sp 3 -hybridization, prevail at 316-750 °C). FeCl crystalline hydrate. 6H 2 O has the structure Cl 2H 2 O. It is highly soluble in water, the solution is yellow; highly hydrolyzed at the cation. Decomposes in hot water, reacts with alkalis. Weak oxidizing and reducing agent.
It is used as a chlorine agent, a catalyst in organic synthesis, a mordant for dyeing fabrics, a coagulant for drinking water purification, an etchant for copper plates in electroplating, and a component of hemostatic drugs.
Equations of the most important reactions:
FeCl 3 6H 2 O=Cl + 2H 2 O (37 °C)
2(FeCl 8 6H 2 O) = Fe 2 O 3 + 6HCl + 9H 2 O (above 250 °C)
FeCl 3 (10%) + 4H 2 O = Cl - + + (yellow)
2FeCl3 (conc.) + 4H 2 O = + (yellow) + - (bc.)
FeCl 3 (dil., conc.) + 2H 2 O → FeCl (OH) 2 ↓ + 2HCl (100 °C)
FeCl 3 + 3NaOH (diluted) = FeO(OH)↓ + H 2 O + 3NaCl (50 °C)
FeCl 3 + 3(NH 3 H 2 O) (conc., horizontal) =FeO(OH)↓+H 2 O+3NH 4 Cl
4FeCl 3 + 3O 2 (air) = 2Fe 2 O 3 + 3Cl 2 (350-500 °C)
2FeCl 3(p) + Cu→ 2FeCl 2 + CuCl 2
Ammonium chloride N H 4 Cl . Oxygen-free salt, technical name is ammonia. White, volatile, thermally unstable. Highly soluble in water (with a noticeable endo-effect, Q = -16 kJ), hydrolyzes at the cation. Decomposes by alkalis when the solution is boiled, transfers magnesium and magnesium hydroxide into solution. Conmutates with nitrates.
Qualitative reaction for the NH 4 + ion - the release of NH 3 when boiled with alkalis or when heated with slaked lime.
It is used in inorganic synthesis, in particular to create a weakly acidic environment, as a component of nitrogen fertilizers, dry galvanic cells, when soldering copper and tinning steel products.
Equations of the most important reactions:
NH 4 Cl (t) ⇌ NH 3 (g) + HCl (g) (above 337.8 °C)
NH 4 Cl + NaOH (saturated) = NaCl + NH 3 + H 2 O (100 °C)
2NH 4 Cl (T) + Ca(OH) 2 (t) = 2NH 3 + CaCl 2 + 2H 2 O (200°C)
2NH 4 Cl (conc.) + Mg = H 2 + MgCl 2 + 2NH 3 (80°C)
2NH 4 Cl (conc., horizontal) + Mg(OH) 2 = MgCl 2 + 2NH 3 + 2H 2 O
NH + (saturated) + NO - 2 (saturated) = N 2 + 2H 2 O (100°C)
NH 4 Cl + KNO 3 = N 2 O + 2H 2 O + KCl (230-300 °C)
Receipt: interaction of NH 3 with HCl in the gas phase or NH 3 H 2 O with HCl in solution.
Calcium hypochlorite Ca(C l O) 2 . Hypochlorous acid salt HClO. White, decomposes when heated without melting. It is highly soluble in cold water (a colorless solution is formed), hydrolyzes at the anion. Reactive, completely decomposes with hot water and acids. Strong oxidizing agent. When standing, the solution absorbs carbon dioxide from the air. Is the active component chlorine (bleach) lime - mixtures of uncertain composition with CaCl 2 and Ca(OH) 2. Equations of the most important reactions:
Ca(ClO) 2 = CaCl 2 + O 2 (180 °C)
Ca(ClO) 2(t) + 4HCl (conc.) = CaCl + 2Cl 2 + 2H 2 O (80 °C)
Ca(ClO) 2 + H 2 O + CO 2 = CaCO 3 ↓ + 2HClO (in the cold)
Ca(ClO) 2 + 2H 2 O 2 (diluted) = CaCl 2 + 2H 2 O + 2O 2
Receipt:
2Ca(OH) 2 (suspension) + 2Cl 2 (g) = Ca(ClO) 2 + CaCl 2 + 2H 2 O
Potassium chlorate KS lO 3 . The salt of chloric acid, HClO 3, is the most famous salt of oxygen-containing chlorine acids. Technical name - Berthollet's salt(named after its discoverer C.-L. Berthollet, 1786). White, melts without decomposition, decomposes upon further heating. It is highly soluble in water (a colorless solution is formed), there is no hydrolysis. Decomposes with concentrated acids. Strong oxidizing agent during fusion.
It is used as a component of explosive and pyrotechnic mixtures, match heads, and in the laboratory as a solid source of oxygen.
Equations of the most important reactions:
4KlO 3 = ZKlO 4 + KCl (400 °C)
2KlO 3 = 2Kl + 3O 2 (150-300 °C, cat. MPO 2 )
KClO 3(T) + 6HCl (conc.) = KCl + 3Cl 2 + ZH 2 O (50-80 °C)
3КlO 3(T) + 2Н 2 SO 4 (conc., horizontal) = 2СlO 2 + КСlO 4 + Н 2 O + 2КНSO 4
(chlorine dioxide explodes in light: 2ClO2(G)= Cl 2 + 2O 2 )
2KlO 3 + E 2(ext.) = 2KEO 3 + Cl 2 (in section NNO 3 , E = Br, I)
KClO 3 +H 2 O→H 2 +KClO 4 (Electrolysis)
Receipt KClO 3 in industry - electrolysis of a hot KCl solution (the KClO 3 product is released at the anode):
KCl + 3H 2 O →H 2 + KClO 3 (40-60 °C, Electrolysis)
Potassium bromide KV r . Oxygen-free salt. White, non-hygroscopic, melts without decomposition. Highly soluble in water, no hydrolysis. Reducing agent (weaker than
Qualitative reaction for the Br ion - displacement of bromine from the KBr solution with chlorine and extraction of bromine into an organic solvent, for example CCl 4 (as a result, the aqueous layer becomes discolored, the organic layer turns brown).
It is used as a component of etchants for metal engraving, a component of photographic emulsions, and a medicine.
Equations of the most important reactions:
2KBr (t) + 2H 2 SO 4 (CONC., hor.) + MnO 2 (t) = Br 2 + MnSO 4 + 2H 2 O + K 2 SO 4
5Вr - + 6Н + + ВrО 3 - = 3Вr 2 + 3Н 2 O
Вr — + Аg + =АgВr↓
2КВr (р) + Сl 2(Г) = 2КСl + Вг 2(р)
KBr + 3H 2 O→3H 2 + KVrO 3 (60-80 °C, electrolysis)
Receipt:
K 2 CO 3 + 2НВr = 2KVr+ CO 2 + H 2 O
Potassium iodide K I . Oxygen-free salt. White, non-hygroscopic. When stored in light it turns yellow. Highly soluble in water, no hydrolysis. Typical reducer. An aqueous solution of KI dissolves I2 well due to complexation.
High quality reaction to ion I - displacement of iodine from the KI solution by a lack of chlorine and extraction of iodine into an organic solvent, for example CCl 4 (as a result, the aqueous layer becomes discolored, the organic layer turns purple).
Equations of the most important reactions:
10I — + 16Н + + 2МnO 4 — = 5I 2 ↓ + 2Мn 2+ + 8Н 2 O
6I - + 14Н + + Сr 2 O 7 2- =3I 2 ↓ + 2Сr 3+ + 7Н 2 O
2I - + 2H + + H 2 O 2 (3%) = I 2 ↓+ 2H 2 O
2I - + 4H + + 2NO 2 - = I 2 ↓ + 2NO + 2H 2 O
5I - + 6H + + IO 3 - = 3I 2 + 3H 2 O
I - + Ag + = AgI (yellow.) ↓
2KI (r) + Cl 2(r) (week) = 2Кl + I 2 ↓
KI + 3H 2 O + 3Cl 2(p) (ex.) = KIO 3 + 6HCl (80°C)
KI (P) + I 2(t) = K) (P) (cor.) (“iodine water”)
KI + 3H 2 O→ 3H 2 + KIO 3 (electrolysis, 50-60 °C)
Receipt:
K 2 CO 3 + 2HI = 2 KI+ CO 2 + H 2 O
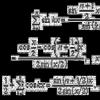
 All chlorine oxides have a pungent odor, are thermally and photochemically unstable, and are prone to explosive decomposition. +1 Cl 2 O T. Pl o. C T. kip °C -120.6 +3 +4 +4 +5 +6 +7 Cl 2 O 3 Cl. O 2 Cl 2 O 4 Cl 2 O 5 Cl 2 O 6 Cl 2 O 7 not obtained by HCl. O 2 -117 9.7 2.0 -59 not obtained 44.5 -93.4 203 HCl. O 3 3 87 HCl. O 4 chlorine chloride dissolved chlorine strong very strong hypochlorites chlorates Na. Cl. O 2 KCl. O 3 perchlorates weak medium strength chlorine KCl. O 4
All chlorine oxides have a pungent odor, are thermally and photochemically unstable, and are prone to explosive decomposition. +1 Cl 2 O T. Pl o. C T. kip °C -120.6 +3 +4 +4 +5 +6 +7 Cl 2 O 3 Cl. O 2 Cl 2 O 4 Cl 2 O 5 Cl 2 O 6 Cl 2 O 7 not obtained by HCl. O 2 -117 9.7 2.0 -59 not obtained 44.5 -93.4 203 HCl. O 3 3 87 HCl. O 4 chlorine chloride dissolved chlorine strong very strong hypochlorites chlorates Na. Cl. O 2 KCl. O 3 perchlorates weak medium strength chlorine KCl. O 4
 § All compounds with chlorine in positive degrees are very strong oxidizing agents. § The most strongly oxidizing properties are expressed in hypochlorous acid, although it is weak and unstable. § Free oxygen-containing chlorine acids are unstable and, except for perchloric acid, exist only in solution. All of them are strong oxidizing agents. § The strength of acids and their oxidizing properties are different concepts. § In the series HCl. O - HCl. O 2 - HCl. O 3 - HCl. O 4 stability and strength of acids increases, and reactivity decreases.
§ All compounds with chlorine in positive degrees are very strong oxidizing agents. § The most strongly oxidizing properties are expressed in hypochlorous acid, although it is weak and unstable. § Free oxygen-containing chlorine acids are unstable and, except for perchloric acid, exist only in solution. All of them are strong oxidizing agents. § The strength of acids and their oxidizing properties are different concepts. § In the series HCl. O - HCl. O 2 - HCl. O 3 - HCl. O 4 stability and strength of acids increases, and reactivity decreases.
 Ratio of halogens to water ü 2 F 20 + 2 H 2 O− 2 → 4 HF + O 2 interaction, F 2 - oxidizing agent, ü Cl 20 + H 2 O ↔ HCl+1 O + HCl− 1 interaction, Cl 20 - oxidizing agent , reducing agent; reaction - disproportionation, ü Br 20 + H 2 O ↔ HBr +1 O + HBr - 1 is highly soluble, practically no interaction occurs; Br 20 – oxidizing agent, reducing agent; reaction – disproportionation, ü I 2 + H 2 O ≠ poorly soluble, practically no interaction occurs; ü At 2 + H 2 O ≠ poorly soluble, practically no interaction occurs
Ratio of halogens to water ü 2 F 20 + 2 H 2 O− 2 → 4 HF + O 2 interaction, F 2 - oxidizing agent, ü Cl 20 + H 2 O ↔ HCl+1 O + HCl− 1 interaction, Cl 20 - oxidizing agent , reducing agent; reaction - disproportionation, ü Br 20 + H 2 O ↔ HBr +1 O + HBr - 1 is highly soluble, practically no interaction occurs; Br 20 – oxidizing agent, reducing agent; reaction – disproportionation, ü I 2 + H 2 O ≠ poorly soluble, practically no interaction occurs; ü At 2 + H 2 O ≠ poorly soluble, practically no interaction occurs
 Chlorine oxides Comparison parameter Chlorine oxide (I) chlorine (IV) State of aggregation at n. u. , color Brownish-yellow gas; at t°
Chlorine oxides Comparison parameter Chlorine oxide (I) chlorine (IV) State of aggregation at n. u. , color Brownish-yellow gas; at t°
 Comparison parameter Chlorine oxide (I) Chlorine oxide (IV) Chlorine oxide (VII) Thermal stability Thermally unstable, decomposes in light Thermally very unstable Slowly decomposes at room temperature The most stable chlorine oxide, decomposes when heated to 120 ° C Toxicity Toxic, affects the respiratory tract routes Toxic Highly toxic Toxic Relation to water Soluble well, interact with water
Comparison parameter Chlorine oxide (I) Chlorine oxide (IV) Chlorine oxide (VII) Thermal stability Thermally unstable, decomposes in light Thermally very unstable Slowly decomposes at room temperature The most stable chlorine oxide, decomposes when heated to 120 ° C Toxicity Toxic, affects the respiratory tract routes Toxic Highly toxic Toxic Relation to water Soluble well, interact with water
 Methods for obtaining chlorine oxides Chlorine oxide Name of method, UHR Chlorine oxide (I) Interaction of mercury oxide (II) with chlorine at 0°C: Hg. O(solid) + 2 Cl 2(gas) → Hg. Cl 2 + Cl 2 O Chlorine oxide (IV) 1) Reaction of potassium chlorate with oxalic acid: KCl. O 3 + H 2 C 2 O 4 → K 2 CO 3 + 2 Cl. O 2 + CO 2 + H 2 O (laboratory method); 2) Passing sulfur dioxide SO 2 into an acidified solution of sodium chlorate: 2 Na. Cl. O 3 + SO 2 + H 2 SO 4 = 2 Na. HSO 4 + 2 Cl. O 2 (industrial method) Chlorine (VI) oxide Oxidation of chlorine (IV) oxide with ozone: 2 Cl. O 2 + 2 O 3 = 2 O 2 + Cl 2 O 6 Chlorine oxide (VII) Reaction of perchloric acid with phosphorus anhydride - phosphorus oxide (V): 8 HCl. O 4 + P 4 O 10 → 4 Cl 2 O 7 + 2 H 4 P 2 O 7
Methods for obtaining chlorine oxides Chlorine oxide Name of method, UHR Chlorine oxide (I) Interaction of mercury oxide (II) with chlorine at 0°C: Hg. O(solid) + 2 Cl 2(gas) → Hg. Cl 2 + Cl 2 O Chlorine oxide (IV) 1) Reaction of potassium chlorate with oxalic acid: KCl. O 3 + H 2 C 2 O 4 → K 2 CO 3 + 2 Cl. O 2 + CO 2 + H 2 O (laboratory method); 2) Passing sulfur dioxide SO 2 into an acidified solution of sodium chlorate: 2 Na. Cl. O 3 + SO 2 + H 2 SO 4 = 2 Na. HSO 4 + 2 Cl. O 2 (industrial method) Chlorine (VI) oxide Oxidation of chlorine (IV) oxide with ozone: 2 Cl. O 2 + 2 O 3 = 2 O 2 + Cl 2 O 6 Chlorine oxide (VII) Reaction of perchloric acid with phosphorus anhydride - phosphorus oxide (V): 8 HCl. O 4 + P 4 O 10 → 4 Cl 2 O 7 + 2 H 4 P 2 O 7
 Chemical properties of chlorine oxides Cl 2 O – chlorine oxide (I) Cl 2+1 O + H 2 O = 2 HCl+1 O not ORP, Cl 2+1 O + 2 KOH = 2 KCl+1 O + H 2 O not OVR, Cl. O 2 – chlorine oxide (IV) 2 Cl+4 O 2 + H 2 O = HCl+3 O 2 + HCl+5 O 3 ORR, Cl+4 – both a reducing agent and an oxidizing agent 2 Cl+4 O 2 + 2 KOH = KCl+3 O 2 + KCl+5 O 3 + H 2 O ORR, Cl+4 – both a reducing agent and an oxidizing agent Cl 2 O 6 – chlorine oxide (VI) Cl 2+6 O 6 + H 2 O = HCl+ 5 O 3 + HCl+7 O 4 ORR, Cl+6 – both a reducing agent and an oxidizing agent Cl 2+6 O 6 + 2 KOH = KCl+5 O 3 + KCl+7 O 4 + H 2 O ORR, Cl+6 – both a reducing agent and an oxidizing agent Cl 2 O 7 – chlorine oxide (VII) Cl 2+7 O 7 + H 2 O = 2 HCl+7 O 4 is not ORR, but Cl 2+7 O 7 + 2 KOH = 2 KCl+ 7 O 4 + H 2 O not ORR,
Chemical properties of chlorine oxides Cl 2 O – chlorine oxide (I) Cl 2+1 O + H 2 O = 2 HCl+1 O not ORP, Cl 2+1 O + 2 KOH = 2 KCl+1 O + H 2 O not OVR, Cl. O 2 – chlorine oxide (IV) 2 Cl+4 O 2 + H 2 O = HCl+3 O 2 + HCl+5 O 3 ORR, Cl+4 – both a reducing agent and an oxidizing agent 2 Cl+4 O 2 + 2 KOH = KCl+3 O 2 + KCl+5 O 3 + H 2 O ORR, Cl+4 – both a reducing agent and an oxidizing agent Cl 2 O 6 – chlorine oxide (VI) Cl 2+6 O 6 + H 2 O = HCl+ 5 O 3 + HCl+7 O 4 ORR, Cl+6 – both a reducing agent and an oxidizing agent Cl 2+6 O 6 + 2 KOH = KCl+5 O 3 + KCl+7 O 4 + H 2 O ORR, Cl+6 – both a reducing agent and an oxidizing agent Cl 2 O 7 – chlorine oxide (VII) Cl 2+7 O 7 + H 2 O = 2 HCl+7 O 4 is not ORR, but Cl 2+7 O 7 + 2 KOH = 2 KCl+ 7 O 4 + H 2 O not ORR,
 Oxygen-containing chlorine acids Physical properties, methods of preparation Chemical properties - relation to heating, alkali solutions and basic oxides
Oxygen-containing chlorine acids Physical properties, methods of preparation Chemical properties - relation to heating, alkali solutions and basic oxides
 Oxygen-containing acids of chlorine Formula of acid Oxidation state of Cl in acid HCl+1 O HCl+3 O 2 HCl+5 O 3 HCl+7 O 4 +1 +3 +5 +7 Increases Thermal stability Increases Acid strength Increases Very weak acid Partially weak acid dissociates in water Form of existence An acid of medium strength, closer to strong One of the strongest acids dissociates almost irreversibly exist only in solution isolated in free form
Oxygen-containing acids of chlorine Formula of acid Oxidation state of Cl in acid HCl+1 O HCl+3 O 2 HCl+5 O 3 HCl+7 O 4 +1 +3 +5 +7 Increases Thermal stability Increases Acid strength Increases Very weak acid Partially weak acid dissociates in water Form of existence An acid of medium strength, closer to strong One of the strongest acids dissociates almost irreversibly exist only in solution isolated in free form
 Hypochlorous acid is obtained by dissolving chlorine oxide (I) in water (1): (1) Cl 2 O + H 2 O → 2 HCl. O Hypochlorous acid – chlorine water, a solution of chlorine in water. It is obtained in a chlorinator by passing chlorine into water until saturation (1 volume of water dissolves about 2.2 volumes of gaseous chlorine at 20°C) (2): (2) Cl 2 + H 2 O ⇌ HCl. O + HCl HCl formed. O decomposes in light into O 2 and HCl. Chlorine water is a strong oxidizing agent and is used to disinfect water and bleach fabrics.
Hypochlorous acid is obtained by dissolving chlorine oxide (I) in water (1): (1) Cl 2 O + H 2 O → 2 HCl. O Hypochlorous acid – chlorine water, a solution of chlorine in water. It is obtained in a chlorinator by passing chlorine into water until saturation (1 volume of water dissolves about 2.2 volumes of gaseous chlorine at 20°C) (2): (2) Cl 2 + H 2 O ⇌ HCl. O + HCl HCl formed. O decomposes in light into O 2 and HCl. Chlorine water is a strong oxidizing agent and is used to disinfect water and bleach fabrics.
 Chlorous acid An acid solution is obtained from its salts - chlorites Ba(Cl. O 2)2 + H 2 SO 4 → 2 HCl. O 2 + Ba. SO 4↓ And also according to the reaction: 2 Cl. O 2 + H 2 O → HCl. O 2 + HCl. O 3 Chlorous acid is an acid of medium strength, closer to weak. Chlorites are used for bleaching.
Chlorous acid An acid solution is obtained from its salts - chlorites Ba(Cl. O 2)2 + H 2 SO 4 → 2 HCl. O 2 + Ba. SO 4↓ And also according to the reaction: 2 Cl. O 2 + H 2 O → HCl. O 2 + HCl. O 3 Chlorous acid is an acid of medium strength, closer to weak. Chlorites are used for bleaching.
 Hypochlorous acid in aqueous solutions at concentrations below 30% is quite stable in the cold; in more concentrated solutions it decomposes: In 8 HCl. O 3 = 4 HCl. O 4 + 3 O 2 + 2 Cl 2 + 2 H 2 O. Hypochlorous acid is a strong oxidizing agent; oxidizing capacity increases with increasing concentration and temperature, for example, filter paper ignites in 40% acid. Hypochlorous acid is obtained in laboratory conditions by reacting barium chlorate with dilute sulfuric acid: Ba(Cl. O 3)2 + H 2 SO 4 = Ba. SO 4↓+ 2 HCl. O 3.
Hypochlorous acid in aqueous solutions at concentrations below 30% is quite stable in the cold; in more concentrated solutions it decomposes: In 8 HCl. O 3 = 4 HCl. O 4 + 3 O 2 + 2 Cl 2 + 2 H 2 O. Hypochlorous acid is a strong oxidizing agent; oxidizing capacity increases with increasing concentration and temperature, for example, filter paper ignites in 40% acid. Hypochlorous acid is obtained in laboratory conditions by reacting barium chlorate with dilute sulfuric acid: Ba(Cl. O 3)2 + H 2 SO 4 = Ba. SO 4↓+ 2 HCl. O 3.
 Perchloric acid Anhydrous perchloric acid is obtained by reacting sodium or potassium perchlorates with concentrated sulfuric acid or aqueous solutions of perchloric acid with oleum, as well as by reacting chlorine oxide (VII) with water: KCl. O 4 + H 2 SO 4 → KHSO 4 + HCl. O 4 Cl 2 O 7 + H 2 O → 2 HCl. O 4
Perchloric acid Anhydrous perchloric acid is obtained by reacting sodium or potassium perchlorates with concentrated sulfuric acid or aqueous solutions of perchloric acid with oleum, as well as by reacting chlorine oxide (VII) with water: KCl. O 4 + H 2 SO 4 → KHSO 4 + HCl. O 4 Cl 2 O 7 + H 2 O → 2 HCl. O 4
 Thermal stability of acids - relation to heating Perchloric acid (HCl. O 4) Ø Can be isolated in free form; Ø When moderately heated with phosphoric anhydride, § 2 HCl decomposes. O 4 + P 2 O 5 = Cl 2 O 7 + 2 HPO 3 Hypochlorous acid (HCl. O 3) Ø When slightly heated, § 8 HCl decomposes. O 3 = 4 HCl. O 4 + 3 O 2 + 2 Cl 2 + 2 H 2 O Chlorous acid (HCl. O 2) Ø Very unstable, decomposes at room temperature in the light § 4 HCl. O 2 = HCl + HCl. O 3 + 2 Cl. O 2 + H 2 O Hypochlorous acid (HCl. O) Ø 2 HCl. O = 2 HCl + O 2 (under the influence of light)
Thermal stability of acids - relation to heating Perchloric acid (HCl. O 4) Ø Can be isolated in free form; Ø When moderately heated with phosphoric anhydride, § 2 HCl decomposes. O 4 + P 2 O 5 = Cl 2 O 7 + 2 HPO 3 Hypochlorous acid (HCl. O 3) Ø When slightly heated, § 8 HCl decomposes. O 3 = 4 HCl. O 4 + 3 O 2 + 2 Cl 2 + 2 H 2 O Chlorous acid (HCl. O 2) Ø Very unstable, decomposes at room temperature in the light § 4 HCl. O 2 = HCl + HCl. O 3 + 2 Cl. O 2 + H 2 O Hypochlorous acid (HCl. O) Ø 2 HCl. O = 2 HCl + O 2 (under the influence of light)
 Relation to alkali solutions When oxygen-containing chlorine acids interact with alkali solutions, an exchange reaction produces a salt of this acid and water. A neutralization reaction occurs. HCl. O2+Na. OH = Na. Cl. O 2 + H 2 O; HCl. O 3 + KOH = KCl. O 3 + H 2 O; Relation to basic oxides When oxygen-containing chlorine acids interact with basic oxides, an exchange reaction produces a salt of this acid and water. 2 HCl. O + Na 2 O = 2 Na. Cl. O + H 2 O; 2 HCl. O 4 + Cu. O = Cu(Cl. O 4)2 + H 2 O
Relation to alkali solutions When oxygen-containing chlorine acids interact with alkali solutions, an exchange reaction produces a salt of this acid and water. A neutralization reaction occurs. HCl. O2+Na. OH = Na. Cl. O 2 + H 2 O; HCl. O 3 + KOH = KCl. O 3 + H 2 O; Relation to basic oxides When oxygen-containing chlorine acids interact with basic oxides, an exchange reaction produces a salt of this acid and water. 2 HCl. O + Na 2 O = 2 Na. Cl. O + H 2 O; 2 HCl. O 4 + Cu. O = Cu(Cl. O 4)2 + H 2 O
In table 16.12 shows the systematic and traditional names of oxygen-containing chlorine acids and their salts. The higher the oxidation state of chlorine in these acids, the higher their thermal stability and acid strength:
5 are strong acids, and 6 is one of the strongest among all known acids. The remaining two acids only partially dissociate in water and
Table 16.12. Oxygen-containing chlorine acids and their anions
exist in aqueous solution mainly in molecular form. Among the oxygen-containing chlorine acids, only 7 can be isolated in free form. Other acids exist only in solution.
The oxidizing ability of oxygen-containing acids of chlorine decreases with increasing oxidation degree:
8 are especially good oxidizing agents. For example, acidic solution 9:
1) oxidizes iron (II) ions to iron (III) ions:
2) decomposes in sunlight to form oxygen:
3) when heated to approximately 75 °C, it disproportionates into chloride ions and chlorate 10-ions:
Salts of oxygen-containing chlorine acids
These salts are usually more stable than the acids themselves. The exception is solid chlorate salts (III), which detonate when heated and upon contact with flammable materials. In solutions, the oxidizing capacity of oxygen-containing chlorine salts is greater, the greater the oxidation state of chlorine in these salts. However, they are not as good oxidizing agents as the corresponding acids. Sodium and potassium salts 11 are of great industrial importance. Their production and applications are described in the next section. Potassium chlorate (V) is usually used for laboratory production of oxygen, in the presence of oxide 12 as a catalyst:
When this salt is heated to a lower temperature in the absence of a catalyst, 13potassium is formed:
Potassium iodate (V) 14 potassium 15 are strong oxidizing agents and are used as oxidizing agents in quantitative analysis.
So, let us repeat once again 1. The properties of the halides of various elements, when moving from left to right within one period, change as follows: a) the nature of the chemical bond becomes more and more covalent and less and less ionic; b) aqueous solutions of halides become increasingly acidic due to hydrolysis. 2. The properties of different halides of the same element when moving to the lower part of group VII change as follows: a) the nature of the chemical bond of the halides becomes more and more covalent: b) the bond strength in the hydrogen halide molecules decreases; c) the acidity of hydrohalic acids decreases; d) the ease of oxidation of hydrogen halides increases. 3. As the degree of oxidation of a halogen increases, the following changes occur: a) the thermal stability of its oxygen-containing acids increases; b) the acidity of its oxygen-containing acids increases; c) the oxidizing ability of its oxygen-containing acids decreases; d) the oxidizing ability of salts of its oxygen-containing acids increases. 4. Halides can be obtained by direct synthesis from their constituent elements. 5. To obtain hydrogen halides, the reaction of displacement from a halide salt with a less volatile acid can be used. 6. Anomalous properties of fluorine compounds: a) silver fluoride is soluble in water, and calcium fluoride is insoluble; b) hydrogen fluoride has abnormally high melting and boiling points; c) an aqueous solution of hydrogen fluoride has low acidity; d) fluorine exhibits only one stable oxidation state. Other halogens exhibit multiple oxidation states, which is explained by the promotion of their 16 electrons into readily accessible 17 low energy orbitals.
===============================================================================
31. Oxygen. Production and properties of oxygen. Allotropy of oxygen. Ozone, its properties. Ozone in nature. Oxygen is an element with serial number 8, its relative atomic mass = 15.999. It is located in the second period, in the main subgroup of group 6.
In most of its compounds, oxygen has an oxidation state of -2. In hydrogen and metal peroxides (H2O2, Na2O, CaO2, etc.), the oxidation state of oxygen is -1. There is only one compound in which oxygen has a positive oxidation state of +2 - this is oxygen fluoride OF2 (fluorine is the only element whose EO is greater than the EO of oxygen, which is 3.5). Ordinary oxygen O2 is a colorless and odorless gas, heavier than air. Slightly soluble in water. Receipt. Laboratory methods O2 production is quite numerous. 1. Dilution of berthollet salt (potassium chlorate) when heated in the presence of manganese(IV) oxide as a catalyst: 2KClO3(t)(MnO2)=2KCl + 3O2
2. Thermal decomposition of potassium permanganate: 2KMnO4(t)=K2MnO4 + MnO2 + O2
3. Thermal decomposition of alkali metal nitrates, for example: 2NaNo3(t) = 2NaNO2 + O2 4. Catalytic decomposition of hydrogen peroxide: 2H2O2(MnO2) = 2H2O + O2
5. Interaction of alkali metal peroxides with carbon dioxide: 2Na2O2 + 2CO2 = 2NaCO3 + O2 6. Electrolysis of aqueous solutions of alkalis or salts of oxygen-containing acids. The essence of the processes occurring in this case comes down to the decomposition of water under the influence of electric current: 2H2O (electrolysis) = 2H2 + O2
In industry, oxygen is obtained from air. Chemical properties.
Oxygen forms compounds with all chemical elements, except light inert gases (He, ne, Ar), and it interacts directly with all simple substances, except fluorine, chlorine, gold and platinum metals. In all reactions, O2 plays the role of an oxidizing agent. When oxygen interacts with simple substances - metals and non-metals - oxides are usually formed; for example: 4Li+O2=2LiO2 4P+5O2(60 degrees)=2P2O5 Almost all reactions involving O2 are exothermic, with rare exceptions; for example: N2+O2=2NO-Q Oxygen can exist in the form of two allotropic modifications: oxygen O2 and ozone O3. Allotropy (from the Greek allos - another and tropos - image, method) is associated either with a different number of atoms in a molecule, or with structure. When comparing the physical properties of oxygen and ozone, it is advisable to remember that these are gaseous substances that differ in density (ozone is 1.5 times heavier than oxygen), melting and boiling points. Ozone dissolves better in water. Oxygen under normal conditions is a gas, colorless and odorless, ozone is a blue gas with a characteristic pungent but pleasant odor. There are also differences in chemical properties.
Ozone is more chemically active than oxygen. The activity of ozone is explained by the fact that its decomposition produces an oxygen molecule and atomic oxygen, which actively reacts with other substances. For example, ozone easily reacts with silver, while oxygen does not combine with it even when heated: But at the same time, both ozone and oxygen react with active metals, for example with potassium K. Ozone is produced according to the following equation: The reaction occurs with the absorption of energy when an electrical discharge passes through oxygen, for example during a thunderstorm, when lightning flashes. The reverse reaction occurs under normal conditions, since ozone is an unstable substance. In nature, ozone is destroyed by gases emitted into the atmosphere, such as freons, during human anthropogenic activities. The result is the formation of so-called ozone holes, i.e. breaks in the thinnest layer consisting of ozone molecules.
Chemical properties: ozone is a strong oxidizing agent, it oxidizes all metals, including gold - Au and platinum - Pt (and platinum group metals). Ozone acts on a shiny silver plate, which is instantly covered with black silver peroxide – Ag2O2; paper soaked in turpentine ignites, metal sulfur compounds are oxidized to sulfuric acid salts; many dyes become discolored; destroys organic substances - in this case, the ozone molecule splits off one oxygen atom, and ozone turns into ordinary oxygen. As well as most non-metals, it converts lower oxides into higher ones, and sulfides of their metals into their sulfates: Potassium iodide oxidizes ozone to molecular iodine: But with hydrogen peroxide H2O2, ozone acts as a reducing agent: Chemically, ozone molecules are unstable - ozone can spontaneously decompose into molecular oxygen:
Being in nature: In the atmosphere, ozone is formed during electrical discharges. Application: being a strong oxidizing agent, ozone destroys various types of bacteria, therefore it is widely used for water purification and air disinfection, and is used as a whitening agent.
================================================================================
32) . Hydrogen peroxide, its structure and properties.