D-aspartic acid. Aspartic acid mechanism of action and regimen of administration L aspartic acid chemical properties
Aspartic acid(eng. D-Aspartic Acid, abbreviated as DAA) is a non-essential amino acid that is encoded by the genetic code in the human body. Other names: aspartate, or aminosuccinic acid. It can be found in all living organisms, both in the composition of protein compounds and in free form. Dietary supplements with this substance are necessary for the normal functioning of several organ systems. Aspartic acid plays the role of a neurotransmitter in the central nervous system, and also stimulates the production of a group of important hormones.
Aspartic acid: synthesis, formation
Synthesis process aspartic acid a living organism occurs by converting the essential amino acid threonine into an isomer of another amino acid - homoserine with further oxidative reactions.
The substance was first discovered in the early 19th century. Scientists Etienne Ossiana Henry and Auguste-Arthur Plisson obtained acid as a result of a substance interaction reaction asparagine with water in the presence of strong mineral acids. Pre asparagine was isolated from asparagus juice by boiling. A few years later, it was recognized as the most important component of proteins.
In modern production, the amino acid is obtained by the condensation of several esters (acetaminomalonic and chloroacetic acid). The carboxyl group is cleaved from the obtained products, followed by hydrolysis.
To make up for the lack aspartic acid in the body, you can eat some foods: meat, cane, asparagus, oatmeal, avocados, soybeans. In this food, the amino acid is contained in too small an amount, so only dietary supplements with a high concentration of the substance are suitable for medicinal purposes.
Aspartic acid: formula
Formula aspartic acid was discovered in 1833 by the scientist J. Liebig. It has the form C 4 H 7 NO 4 .
The structural formula of an acid is as follows. You need to know that it has several forms that differ in their structure: L and D forms. The first is found in the human body in large quantities, and the second - only in adults.
Despite the general functions, it is responsible for the synthesis of proteins and the purification of toxic substances, and the D-form normalizes brain functions and stimulates the production of hormones. Such differentiation is not of particular importance, because when the L-form enters the body, it is transformed into the D-amino acid isomer.
Aspartic acid: exchange
Aspartic acid is directly involved in many chemical processes, in particular, the exchange of amino acids in the human body. This affects the composition of proteins, and, consequently, the structure of cells. Also aspartate together with glutamic acid, they bind molecules of highly toxic ammonia and other nitrogenous compounds. As a result of reducing their concentration, the negative impact on the body decreases.
Aspartic acid: composition
The composition of biologically active additives with aspartic acid quite simple: they contain only one component that affects the level of hormones and other indicators. A high concentration of acid allows you to achieve maximum effect in a short period of time.
Depending on the type of production, additional ingredients may be present in the preparations - residual products. For example, wheat, milk, peanuts, herbs and more. They are harmless to the body and are contained in a very small amount, which does not affect the properties of the supplement.
 Aspartic acid: properties
Aspartic acid: properties
Properties aspartic acid widely known to various categories of people: athletes, men and women with sexual problems or cognitive impairment.
To positive properties aspartic amino acid include:
- is a component of proteins that serve as material for building cells;
- normalizes the work of the endocrine system due to interaction with the hypothalamus;
- promotes the production of somatropin (growth hormone) and the main sex hormones of men and women (testosterone, progesterone);
- plays the role of a neurotransmitter in the central nervous system, improves brain function;
- has antibacterial properties;
- necessary for the formation of other amino acids, such as,;
- promotes the movement of mineral compounds that support the normal functioning of RNA and DNA;
- enhances the protective function of the immune system;
- prevents the reproduction of microorganisms and neutralizes their toxic effect due to increased production of antibodies;
- rids the body of the products of nitrogen metabolism, including ammonia;
- protects nerve cells from pathogenic effects;
- struggles with mental disorders, depressed mood;
- increases muscle mass.
It should be noted that it goes through the stage of oxidation in mitochondria, forming the energy necessary for the body. This amino acid is not only a source of energy, but also the most important energy stimulator of the brain.
Aspartic acid: application
Aspartic acid applies in several, completely different cases, such as:
- a depressive state (you can also get rid of it with the help of,);
- cognitive disorders (impaired thinking, memory impairment);
- erectile dysfunction and decreased sexual activity;
- for the purpose of building muscle for athletes;
- hormonal imbalance (hormone deficiency);
- CNS disorders;
- for the treatment of cardiovascular diseases;
- visual impairment (nearsightedness, nyctalopia);
- toxic contamination of the liver;
- chronic fatigue syndrome;
- postoperative period or recovery after a serious illness.
It is important that with an insufficient amount of amino acids in the body, patients experience memory impairment, a depressed state and an unwillingness to engage in vigorous activity. However, with an increased amount of a substance in the body, a person feels nervous and aggressive, unable to control emotions. There are even changes in the density of the blood, which often causes blood clots in the blood vessels.
Usually application aspartic acid essential for all people over 40. At this age, even in a healthy person, its level begins to decrease. Therefore, in order to prevent possible diseases, it is advisable to take acid without a good reason.
 Aspartic acid: testosterone
Aspartic acid: testosterone
Scientists have proven that it regulates the synthesis of testosterone, the main male sex hormone. This biologically active substance affects not only the production of spermatozoa, but also the development of muscles and bones, it can set the emotional background.
For the first time, the increase in this hormone was studied as a result of studies on rats. After a few years, the effectiveness aspartic acid was confirmed once again, but in this case, the substance was taken by people. So, after 12 days of daily intake aspartate the level of the male hormone increased by almost half, which was a landmark discovery in the world of medicine.
Also, the amino acid is able to increase the production of its own gonadotropin. This hormone is produced in the body of a pregnant woman, as well as in the anterior pituitary gland in all people. It has a stimulating effect on the synthesis of male germ cells and is necessary for the normal development of the organs of the reproductive system.
For male hormones, it is useful to take and. With an increased level of testosterone is prescribed.
Aspartic acid: libido, erection
To restore sexual desire and normalize an erection, you need. This is due to the fact that it increases the natural production of sex hormones that significantly affect sexual function.
A decrease in libido, as well as an increase in the mammary gland in men and prostate diseases occur due to an increase in estrogen levels. This happens extremely rarely, but significantly disrupts sexual life. It will help to deal with it. Both men and women can take the substance. Over time, sexual desire will resume and the former quality of life will be restored.
Aspartic acid: bodybuilding
Aspartic acid often used in bodybuilding by professional athletes to quickly build muscle mass. With its help, they regulate the work of the endocrine system, namely the production of growth hormone and testosterone, a hormonal muscle growth factor. Often athletes take the more well-known herbal dietary supplement.
Aspartic acid necessary not only for those who want to have a relief inflated body. Its regular use will help to achieve high results in powerlifting, where the athlete's strength indicators play the main role. During the period of preparation for competitions or active training, this substance is taken without fail. The first positive changes will be manifested in the loss and increase in endurance during active physical exertion.
How does it help you? Your feedback is very important for beginners and people suffering from similar ailments!
Was the article helpful?
Choose to rate!

Mechanism of action and regimen
And spartic acid (also aspartate or DAA) is a substance present in the organisms of all living beings and involved in the functioning of the nervous system. This amino acid is responsible for the transmission of nerve impulses through neurons and stimulates the production of hormones: luteinizing, follicle-stimulating and growth hormone.
In the body of men, this substance, formed in the testicles, slightly increases the level. This ability has become fundamental for the use of acid in bodybuilding, since the level of muscle mass of a weightlifter depends on the concentration of testosterone in the blood.
Mechanism of action and functions
Aspartic acid is an alpha-amino acid with the chemical formula HOOCCH(NH2)CH2COOH.
Modern biological studies have confirmed its ability to influence the functioning of the endocrine system. DAA stimulates the production of gonadotropin, which helps the synthesis of testosterone, the main anabolic in heavy weight athletes.
Western bodybuilders and powerlifters actively use aspartate to improve performance: increase strength indicators, and muscle volumes. In Russia, dietary supplements are just beginning to gain popularity. By taking this acid, you can get:
- increased production of testosterone;
- increase in the amount of gonadotropin;
- fast ;
- increase in strength indicators and rapid recovery after training.
D-aspartic acid has the closest substance in composition - L-aspartic acid. It does not have anabolic properties, so you need to be careful when buying supplements in a pharmacy.
The body is able to independently produce asparagine in the optimal amount for the average person. You can get it from food, especially important:

Even before the use of supplements with D-aspartic acid, preparations based on it were produced in Italy - as a fertility drug under the name DADAVIT.
The interest of athletes in the amino acid has increased significantly in recent years, so preparations based on it are widely represented abroad. In Russia, of the certified products, only DAA Ultra can be noted - an additive from a trusted sports nutrition manufacturer Trec Nutrition.
Acid supplements are labeled D-Aspartic Acid. The following dietary supplements are popular:

- Powders: DAA D-Aspartic Acid, KFD Premium DAA, Primaforce DAA - 1 serving (3 g) contains 3000 mg of the substance.
- Pills: DAA Xtreme Prolact block.
- Capsules: AI Sports Nutrition, D-Aspartic Acid. One capsule contains D-aspartic acid calcium chelate - 780 mg, B6 - 0.5 mg (25% of the daily requirement), (as cyanocobalamin) - 1.5 mcg (25% of the norm), folic acid (folate) - 100 mcg (25% of the norm).
Athletes note that the most convenient forms are capsules and tablets - the powder is not very palatable and does not dissolve well. If the dietary supplement is purchased in the form of the latter, it is better to turn it into a suspension - dilute it in 50 ml of juice, and after taking it, drink it with water.
There is evidence that DAA sensitivity varies with age, endocrine status, regimen, and athlete. In sports nutrition, the amino acid can be combined with your favorite special supplements, however, the interval between taking different substances should be 15-20 minutes.

Aspartic acid increases the level of prolactin in the blood, so after a course of steroids, you need to take a break to minimize their effect - only after that you can start the next course of taking DAA.
It is worth being careful - aspartic acid cannot be combined with other drugs that stimulate the production of testosterone. Such combinations can provoke hormonal failure.
Admission rules

The maximum effect from the effects of the substance can be achieved using a cyclic scheme: 3 weeks of admission with a break of 2 weeks. Efficiency is evaluated at the end of the first cycle.
The initial dose of the substance is 3 g / day. They are divided into three parts and taken on an empty stomach in the morning, afternoon and evening. If necessary, you can increase the dose to 20 g / day.
It is necessary to take DAA in the maximum doses allowed by the instructions only in some cases - most athletes get a positive result from taking 5-10 g / day. A course lasting less than 3 weeks in a row will not be very effective, and prolonging the course leads to a decrease in the effectiveness of the acid - testosterone production decreases sharply with prolonged use.
Contraindications and side effects
Direct indications for taking amino acids:

Unlike anabolics and steroids, aspartic acid does not suppress the natural production of hormones, but stimulates the process.
After discontinuation of the drug, the body continues to synthesize testosterone in a natural way. Despite this, taking aspartic acid is contraindicated:

- women, since the effect of acid on their body has not yet been studied;
- men under 21;
- with increased production of testosterone, dihydrosterone, estrogen;
- with violations of the hormonal system;
- persons suffering from diseases of the liver, kidneys, diabetes.
Acid intake can lead to some side effects:
- Elevated levels of dihydrosterone cause acne and hair loss.
- Increased estrogen production can lead to gynecomastia, flatulence, inflammation of the prostate and decreased libido.
- An increase in androgens in the blood, which causes an increase in aggression.
Aspartic acid inhibits the production of melatonin, so it is not recommended to use it in the evening. It is better to distribute the daily dose in such a way that the reception ends no later than 17-18 hours.
An overdose of amino acids (more than 20 g / day) can cause severe headaches, depression, gastrointestinal upset, a drop in blood pressure and blood clots.
D-aspartic acid is used by bodybuilders to achieve high results in strength training. The main condition is to use the supplement wisely and control its effect on the body.
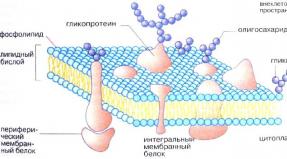
Aspartic acid (abbreviated as Asp or D) is an alpha amino acid with the chemical formula HOOCCH(NH2)CH2COOH. The carboxylate anion, salt or ester of aspartic acid, is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids that are part of the building blocks of proteins. Its codons are GAU and GAC.
Aspartic acid, along with glutamic acid, are classified as acidic with a pKa of 3.9, however, in the peptide, the pKa is highly dependent on various conditions. It is not uncommon for PKa to reach a value of 14. Aspartate is quite common in biosynthesis. As with all amino acids, the presence of acidic protons depends on the environment of the chemical residue and the pH of the solution.
Opening
In 1827, Plisson first discovered aspartic acid in , a substance that was isolated from asparagus juice in 1806, by boiling with a base.
Forms of aspartic amino acid
There are two forms or enantiomers of aspartic acid. The name "aspartic acid" can refer to either enantiomer, or a mixture of the two. Of these two forms, only one, "L-aspartic acid", is incorporated directly into proteins. The biological role of its counterpart, "D-aspartic acid", is more limited. If we end up with either one or the other form of acid through enzymatic synthesis, most chemical syntheses will end up with both forms, "DL-aspartic acid", known as a racemic mixture.
Role in amino acid biosynthesis
Aspartate is a non-essential amino acid in mammals and is produced from oxaloacetate by transamination. It can also be obtained from ornithine and citrulline in the urea cycle. In plants and microorganisms, aspartate is the precursor of several amino acids, including four essential for humans:, and. The conversion of aspartate to these others begins with the conversion of aspartate to its "semialdehyde" O2CCH(NH2)CH2CHO. Asparagine is produced from aspartate via transamidation:
-O2CCH (NH2) CH2CO2-+ GC (O) NH 3 + O2CCH (NH2) CH2CONH3 + + GC (O)
(Where GC(O)NH2 and GC(O)OH are respectively glutamine and glutamic acid)
Other biochemical roles
Aspartate is also a metabolite in the cycle and is involved in gluconeogenesis. It carries reducing equivalents in a malate-aspartate shuttle mechanism that utilizes the ready-made interconversion of aspartate and oxaloacetate, an oxidized (dehydrogenated) malic acid derivative. Aspartate donates one nitrogen atom to the biosynthesis of inosine, a precursor of purine bases. In addition, aspartic acid acts as a hydrogen acceptor in the ATP synthase chain.
Aspartate as a neuroreceptor mediator
Food sources of aspartic acid
Aspartic acid is not an essential amino acid, meaning it can be synthesized from central intermediates in human metabolism. Aspartic acid is found in:
Animal sources: meat, sausage, game
Plant Sources: Seed sprouts, oatmeal, avocado, asparagus, young sugar cane, and sugar beet molasses.
Dietary supplements such as aspartic acid itself or salts (such as magnesium aspartate)
Aspartame sweetener (NutraSweet, Equal, Canderel, etc.)
Chemical synthesis of aspartic acid
Racemic aspartic acid can be synthesized from sodium diethyl phthalimidomalonate, (C6H4 (CO) 2 NC (CO 2 Et) 2).
The main disadvantage of the above method is that although equimolar amounts of each enantiomer are produced, the body only uses . With the help of biotechnology, it is now possible to use immobilized enzymes to create only the same type of enantiomers due to their stereospecificity. Aspartic acid is synthetically produced using ammonium fumarate and aspartase from E. coli. E. coli normally process aspartic acid as a source of nitrogen, but with excess amounts of ammonium fumarate, a change in the functioning of the enzyme is possible, and thus aspartic acid will be produced in very large quantities, 98.7 mm from 1 M.
(CC(O)=O)C(O)=O]
Aspartate redirects here. It should not be confused with Aspartame, a food sweetener, and Asparkam, a drug.
Aspartic acid (aminosuccinic acid, aspartate, aminobutanedioic acid, 2-aminobutanedioic acid) is an aliphatic amino acid, one of the body's 20 proteinogenic amino acids. It occurs in all organisms in free form and as part of proteins. In addition, it acts as a neurotransmitter in the central nervous system.
Getting aspartate
Physiological role
Aspartic acid:
- present in the body in the composition of proteins and in free form
- plays an important role in nitrogen metabolism
- participates in the formation of pyrimidine bases and urea
- Aspartic acid and asparagine are critical for the growth and reproduction of leukemic cells in some types of lymphocytic leukemia. An enzyme of microbial origin, L-asparaginase, which disrupts the conversion of aspartic acid to asparagine and vice versa, has a strong specific cytostatic effect in these types of leukemia.
Application
The acid itself and its salts are used as components of medicines.
- Asparkam - tablets, the amount of salts - potassium and magnesium asparaginates ( aspar aginates ka Leah and m Agnia), is used in the treatment of cardiovascular disorders.
Write a review on the article "Aspartic acid"
Notes
|
||||||||||||||
An excerpt characterizing Aspartic acid
All his desires were fulfilled this morning; a general battle was given, he participated in it; moreover, he was an orderly under the bravest general; moreover, he went on an assignment to Kutuzov, and perhaps to the sovereign himself. The morning was clear, the horse under it was kind. His heart was full of joy and happiness. Having received the order, he started his horse and galloped along the line. At first he rode along the line of Bagration's troops, who had not yet entered into action and stood motionless; then he drove into the space occupied by Uvarov's cavalry and here he already noticed movements and signs of preparations for the case; having passed Uvarov's cavalry, he already clearly heard the sounds of cannon and cannon fire in front of him. The shooting intensified.In the fresh, morning air there were already heard, not as before at unequal intervals, two or three shots, and then one or two cannon shots, and along the slopes of the mountains, in front of Pracen, the rifts of rifle fire were heard, interrupted by such frequent shots from guns that sometimes several cannon shots no longer separated from each other, but merged into one common roar.
One could see how the smoke of the guns seemed to be running along the slopes, chasing each other, and how the smoke of the guns swirled, blurred and merged one with the other. One could see, by the gleam of bayonets between the smoke, moving masses of infantry and narrow bands of artillery with green boxes.
Rostov, on a hillock, stopped his horse for a moment to examine what was being done; but no matter how he strained his attention, he could neither understand nor make out anything of what was being done: some people were moving there in the smoke, some canvases of troops were moving in front and behind; but why? who? where? could not be understood. This sight and these sounds not only did not arouse in him any dull or timid feeling, but, on the contrary, gave him energy and determination.
“Well, more, give me more!” - he turned mentally to these sounds and again started galloping along the line, penetrating further and further into the area of \u200b\u200bthe troops that had already entered into action.
“I don’t know how it will be there, but everything will be fine!” thought Rostov.
Having passed some kind of Austrian troops, Rostov noticed that the next part of the line (it was the guard) had already entered into action.
"All the better! I'll take a closer look, he thought.
He went almost to the front line. Several riders galloped towards him. These were our Life Lancers, who were returning from the attack in disordered ranks. Rostov passed them, noticed involuntarily one of them in the blood and galloped on.
"I don't care about that!" he thought. Before he had gone a few hundred paces after that, to his left, across from him, appeared throughout the field a huge mass of cavalrymen on black horses, in shiny white uniforms, who trotted straight at him. Rostov set his horse at full gallop in order to get out of the way from these cavalrymen, and he would have left them if they were still walking at the same gait, but they kept gaining speed, so that some horses were already galloping. Rostov became more and more audible to their clatter and rattling of their weapons, and their horses, figures and even faces became more visible. These were our cavalry guards attacking the French cavalry advancing towards them.
Aspartic acid (aminosuccinic acid, aspartate) - aliphatic amino acid, one of the 20 proteinogenic amino acids of the body, usually found in the L-form. It is found in all organisms in free form and as part of proteins, especially in sugar cane and sugar beets. It is an essential amino acid, has an overall negative charge and plays an important role in the synthesis of other amino acids, citric acid and urea cycles. Asparagine, arginine, lysine, methionine, isoleucine, and some nucleotides are synthesized from aspartic acid. In addition, it acts as a neurotransmitter in the central nervous system. Biosynthesis is carried out as a result of the isomerization of threonine to homoserine, followed by its oxidation or as a result of the hydrolysis of asparagine. Aspartate is isolated from protein hydrolysates. Aspartic acid is obtained by condensation of acetaminomalon ester with chloroacetic acid ester followed by hydrolysis and decarboxylation of the condensation products or acid hydrolysis of asparagine. Methods for the isolation and analysis of aspartic acid are based on the insolubility of its calcium and barium salts.
L-isomer is used for the synthesis of peptides, in a mixture with other amino acids - for parenteral nutrition. It was first isolated by G. Riethausen in 1868 from the proteins conglutin and legumin. World production about 250 tons/year (1982)Physiological role and application in medicine
Aspartic acid:
- present in the body as part of proteins and in free form;
- plays an important role in the metabolism of nitrogenous substances;
- participates in the formation of pyrimidine bases and urea;
- Aspartic acid and asparagine are critical for the growth and reproduction of leukemic cells in some types of lymphocytic leukemia;
- the enzyme of microbial origin L-asparaginase, which disrupts the conversion of aspartic acid to asparagine and vice versa, has a strong specific cytostatic effect in these types of leukemia;
- Asparkam - tablets, the amount of salts - potassium and magnesium aspartates (potassium and magnesium aspartates), is used in the treatment of cardiovascular disorders.
Physicochemical characteristics
Protein-protein contacts
Aspartic acid does not contain sulfur atoms, so it cannot form disulfide bonds. It is charged, so it does not form hydrophobic bonds. The following connections are typical for her:
1. Salt bridges
In this case, the amino acid is negatively charged, so it can form salt bridges with positively charged amino acids, such as arginine, lysine, histidine.
DNA-protein contacts
Since aspartic acid is negatively charged, it cannot form hydrophobic bonds with DNA. Moreover, since DNA is also negatively charged, electrostatic forces repel aspartic acid from it. Perhaps that is why it was not possible to find any links between DNA and this amino acid.
Read also...
- Other names: sleepy dope, belladonna, wild berry, wild cherry, rubuha
- Other names: sleepy dope, belladonna, wild berry, wild cherry, rubuha
- Aspartic acid mechanism of action and regimen of administration L aspartic acid chemical properties
- Symptoms and diagnosis of Dupuytren's disease - treatment, surgery and rehabilitation course Mkb 10 Dupuytren's contracture treatment terms