Cardiovascular continuum. The cardiovascular continuum: common causes of many diseases II. Hemodynamic overload of the heart muscle
Cardiovascular diseases (CVD) account for almost half of all deaths on the European continent. Every year in 53 countries - members of the WHO, 4.35 million people die from this pathology, and in the European Union (EU) - 1.9 million people. Coronary heart disease (CHD) prevails in the structure of cardiovascular mortality, accounting for 40%. Of the annual costs of treating cardiovascular diseases in the European Union, which amount to 169 billion euros (average 372 euros per person per year), 27% of this amount is spent on treating patients with coronary heart disease. The lion's share of the money is spent on therapy for the most frequent and formidable complication - chronic heart failure (CHF). Note that the prognosis of the life of people suffering from CHF directly depends on socio-economic conditions: poor people have a 39% higher risk of death than those who are financially secure.
At one time, this determined the priorities in spending on health care authorities in the "old" countries of the European Union, which has borne fruit: morbidity and mortality from coronary heart disease is steadily decreasing here. The same picture is observed in the Scandinavian countries, the USA and Japan, which were once the leaders in mortality from arterial hypertension (AH). At the same time, the number of patients with CHF is growing everywhere and progressively. What is the reason for this growth and is there any opportunity for cardiologists to somehow change the situation?
Epidemiological aspects
In addition to the main reason for this growth - the aging of the population, a considerable contribution, paradoxically, is made by cardiologists in the field of treating their patients. For example, a decrease in mortality from myocardial infarction (MI), an improvement in patient survival lead to an increase in the number of people with left ventricular systolic dysfunction (LV DM), which develops in the postinfarction period in 40% of patients (TRACE), and successful antihypertensive therapy in patients with arterial hypertension - in patients with left ventricular diastolic dysfunction... On the other hand, diastolic dysfunction of the left ventricle appears even faster in patients with arterial hypertension if antihypertensive therapy is inadequate, which is not uncommon. Among specialists, volatile is “ halves rule", Stating that" only half of the patients know that they have arterial hypertension, half of them are treated, and half of them are treated effectively».
Of more than 1 billion patients with arterial hypertension, 7.1 million patients die every year due to unsatisfactory blood pressure control... In 1995, for example, in Great Britain, patients with a newly diagnosed arterial hypertension stopped taking antihypertensive drugs after a few months; in the United States and Spain, 84 and 85% of patients receive antihypertensive drugs, but of these, only 53 and 27% of them effectively control blood pressure. ... According to other data given by prof. MP Savenkov at a meeting of the cardiology section of the Moscow City Society of Physicians on October 18, 2007, in the United States, effective blood pressure control is carried out in 30% of patients, and in Russia - only in 12%.
According to the authoritative Framingham study, carried out in the era of the absence of effective antihypertensive drugs, congestive CHF was the cause of death in 40% of patients with arterial hypertension. Subsequent observations of epidemiologists have confirmed the fact of particular importance arterial hypertension as a risk factor for chronic heart failure... Specifically, according to the 14-year Framingham Study, arterial hypertension by itself or in combination with coronary heart disease preceded clinical manifestations chronic heart failure in 70% of cases in both men and women. With blood pressure above 160/100 mm Hg. Art. the risk of developing CHF is 2 times higher than with blood pressure below 140/90 mm Hg. Art. The attributive (population) risk of developing chronic heart failure in hypertensive patients is 39% for males and 59% for females. For comparison: at stable angina it accounts for 5 and 6%, respectively, for diabetes mellitus 6 and 12%.
Etiopathogenetic aspects
Arterial hypertension as the main risk factor for the development of chronic heart failure has received great attention for many reasons. Back in 1991, well-known scientists V. Dzau and E. Braunwald introduced the term “ cardiovascular continuum". According to this model (Fig. 1), cardiovascular diseases are a sequential chain of events: the start begins with the main risk factors (FR), which include primarily arterial hypertension, dyslipidemia, diabetes mellitus, insulin resistance and smoking... If nothing is done, for example, not to treat arterial hypertension, then sooner or later the patient may get a stroke or acquire coronary heart disease, and then a chain of formidable complications will end in the inevitable development of CHF and death.
In 2001, A. M. Dart and B. A. Kingwell described the second ("Pathophysiological") continuum (Fig. 2), which is a vicious circle that starts with the stage of damage to the vascular endothelium and its dysfunction - this is the root cause of arterial atherosclerosis. Further, the circle is closed by increasing the rigidity of the walls of resistive vessels, which leads to an acceleration of the pulse wave and an increase in pulse pressure, as well as blood pressure in the aorta. As a result, endothelial dysfunction progresses, the risk of atherothrombotic complications increases. According to this model, arterial hypertension is a key factor in accelerating the atherosclerotic process and the appearance of coronary heart disease. The latter is accompanied by ischemic myocardial damage up to the development of myocardial infarction and dysfunction of the heart muscle.
In patients with arterial hypertension, the heart is forced to adapt to working conditions against high resistance of peripheral vessels, which spasm in response to an increase in blood pressure. Sooner or later, the wall of the left ventricle of the heart thickens, which at first is the result of its adaptation. Over time, degenerative changes appear in hypertrophied cardiomyocytes (CMC), collagen accumulates in the interstitial spaces. Already in the early stages of arterial hypertension, left ventricular hypertrophy (LVH) and left ventricular diastolic dysfunction (DD LV). Even mild arterial hypertension increases the risk of LVH by 2-3 times - this is a risk factor for myocardial infarction and ventricular arrhythmias. The occurrence of vascular endothelial dysfunction under conditions of oxidative stress contributes to the accelerated progression of the atherosclerotic process in the vessels, including coronary vessels. This poses a threat of myocardial ischemia and increases the risk of myocardial infarction, which is facilitated by a decrease in left ventricular muscle perfusion due to the presence of its hypertrophy.
If left ventricular diastolic dysfunction is the result of high resistance loading, left ventricular systolic dysfunction results from volume overload. A decrease in tissue perfusion with blood is accompanied by a compensatory activation of neuroendocrine systems, primarily sympathoadrenal (SAS) and RAAS. Hyperactivation of the latter accelerates the progression of chronic heart failure. Note that left ventricular systolic dysfunction occurs in 2% of the population, in 50% of patients, it is asymptomatic, patients are not treated, which worsens their life prognosis.
The main drug approaches to reduce the risk of developing CHF
In the recommendations of the European Society for the Study of Arterial Hypertension and the European Society of Cardiology ( www.escardio.org) it is emphasized that “ the beneficial effect of antihypertensive therapy is due to the achieved decrease in blood pressure, regardless of the agent used, with which this decrease is achieved", And" the main classes of antihypertensive drugs - diuretics, beta-blockers, calcium antagonists (AC), angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists (ARA) - are equally suitable for both initial and maintenance therapy". At the same time, it is recognized that the effectiveness of certain classes of antihypertensive drugs may be greater in some specific patient groups.
Analysis of the results of 12 most significant studies on the treatment of arterial hypertension, which took into account cases of chronic heart failure, showed that antihypertensive therapy reduces their risk by an average of half, while the risk of coronary heart disease - by 16%, strokes - by 38% , LVH - by 35%. The latter is of particular importance, since without prior arterial hypertension it occurs very rarely and at first has a compensatory value.
With the formation of diastolic dysfunction of the left ventricle, when the coronary reserve decreases and various kinds of arrhythmias may appear, the situation is still reversible. From the stage of the onset of left ventricular systolic dysfunction, remodeling of the heart muscle acquires irreversible... Note that LVH doubles the absolute risk of myocardial infarction in the elderly (this is the most common cause of the rapid onset of left ventricular systolic dysfunction), but the highest relative risk of myocardial infarction with LVH is observed in middle-aged people with arterial hypertension.
P. A. Meredith and J. Ostergen, A. U. Klingbeli et al. analyzed the effectiveness of various antihypertensive drugs in terms of their effect on the left ventricular mass. The baseline data for the meta-analysis were the results of 80 studies (n \u003d 3767 patients) of active treatment and 17 - placebo-controlled antihypertensive therapy (n \u003d 346 patients). It has been established that both calcium antagonists and ACE inhibitors have a more pronounced effect on LVH than beta-blockers. At the same time, more and more data indicate that the most effective drugs in this regard are angiotensin II receptor antagonists (CATCH; LIFE). At least today, it can be argued with confidence that their effect is not worse than ACE inhibitors. The experience gained by cardiologists in the treatment of patients with arterial hypertension gives grounds to recommend drugs acting on the RAAS as the main strategy for regression of LVH.
In this regard, the issue of therapeutic tactics in the presence of atrial fibrillation... The latter occurs in every third patient with CHF and carries a risk of increased mortality from cardiovascular causes, especially from cerebral stroke. According to V. Fuster et al., In such patients the risk of ischemic cerebral stroke is 2-7 times higher than in patients without atrial fibrillation. CHF is common reason the occurrence of atrial fibrillation, but with poorly controlled heart rate, atrial fibrillation can lead to the onset and rapid progression of chronic heart failure. In patients with arterial hypertension and LVH, the risk of developing atrial fibrillation is 42% (Manitoba Follow-Up Study :). A significant contribution to the appearance of atrial fibrillation in patients with arterial hypertension is made by RAAS, therefore, from these positions, preference should be given to ACE inhibitors (SOLVD) and angiotensin II receptor antagonists (CHARM :). It has been proven that they are able to influence the process of left atrial remodeling, an increase in which is associated with the onset of atrial fibrillation.
There were reports of effective use statins for the prevention of atrial fibrillation in patients with left ventricular dysfunction, after heart surgery (ARMYDA-3), after cardioversion, as well as in pharmacotherapy for patients with coronary heart disease. Their beneficial effect is explained in terms of their influence on the process of inflammation and antioxidant effect. According to the experience of D. Amar et al., The antifibrillatory effect of statins is also manifested in patients with normal level C-reactive protein (SRB). Note that the relationship between vascular inflammation, CRP levels and the risk of atrial fibrillation is well established.
Of the 1 billion people with hypertension on the planet, 7.1 million die each year as a result of inadequate antihypertensive therapy. Two thirds of deaths are due to cerebral stroke, although it is well known that a decrease in SBP in total by 5 mm Hg. Art. accompanied by a decrease risk of death from stroke by 14%... It seems like a simple task. It can be solved by using almost any of the antihypertensive drugs, such as clonidine. At the same time, the use of the latter, judging by the experience of Finnish colleagues, increases the risk of cerebral stroke. Thus, according to their long-term results of use, not all antihypertensive drugs are the same.
The most convincing data on the prevention of the risk of development and progression of chronic heart failure and its complications were obtained when used in patients with arterial hypertension. aCE inhibitors and angiotensin II receptor antagonistswith organoprotective properties. All of these drugs there is a "class effect" in reducing mortality and mortality from cardiovascular causes in patients with CHF with left ventricular dysfunction; in patients who have undergone AMI with and without left ventricular systolic dysfunction; high coronary risk; suffering from diabetes mellitus (DM) and kidney dysfunction. All of them are highly effective as antihypertensive drugs, although, according to recent data, in terms of their effect on the risk of cardiovascular complications in patients with arterial hypertension, they are comparable to other antihypertensive drugs, which was confirmed after the completion of a number of large programs. In particular, in the studies THOMS, STOP-2, HANE, CAPPP, UKPDS, ALLHAT, significant advantages of some antihypertensive drugs over others in terms of improving the prognosis in patients with arterial hypertension have not been established.
At the same time, even ACE inhibitors are a group of dissimilar chemical compounds, which suggests different efficacy in specific groups of patients. According to J.P. Tsikouris et al., In patients with a history of AMI with left ventricular systolic dysfunction quinapril is much more effective than enalapril in terms of reducing cardiovascular mortality, as well as the level of CRP - this is the most important marker of vascular inflammation and a predictor of the risk of coronary complications.
If trandolapril proved to be an effective drug in terms of improving the prognosis in patients with left ventricular dysfunction after AMI when administered in a relatively small dose, this does not automatically mean that it will be equally effective in patients without left ventricular systolic dysfunction. The fact is that the presence of systolic dysfunction of the left ventricle, as a rule, is a causal factor in the formation of a congestive form of chronic heart failure. In such patients, excessive activation of neurohumoral systems is observed, which may not be the case in individuals without symptoms of left ventricular dysfunction. In this case, these doses of trandolapril may not be effective.
Within the framework of the discussed topic of this article, the conclusions of two large studies - EUROPA and HOPE - are of fundamental importance, despite the difference in design and goals. Application perindopril (EUROPA) in patients with high-risk coronary heart disease, but significantly (40-80%) less than in patients in the HOPE study with ramipril, led to a reduction in the risk of AMI by 24%, and CHF by 39%. This result cannot be construed solely antihypertensive effect perindopril, since out of 12,218 treated patients, arterial hypertension was only in 27% of patients, and the decrease in SBP and DBP was 5 and 2 mm Hg, respectively. Art.
Striking data were obtained in the HYVET study, in which effective antihypertensive therapy ( arifon retard +/- prestarium) in elderly patients led to a 64% reduction in the risk of chronic heart failure. Impressive results have been obtained in the HOPE study in patients who have already had cerebral stroke, in persons with very high coronary risk. Of the 9541 patients over the age of 55, about half of the patients suffered from arterial hypertension. Appointment ramipril led to a relatively small decrease in SBP and DBP (by 3.0 and 1.0 mm Hg, respectively), but the risk of AMI decreased by 20%. At the end of the 4.5-year HOPE study, an additional HOPE / HOPE-TOO was launched with a duration of 2.6 years. Its feature was the comparability of the frequency of ACE inhibitors in the groups of people who received ramipril (72%) and placebo (68%). An additional decrease in the relative risk of AMI was 19%, CHF - 27.8%, which was explained by the peculiarity of the action of the drug itself.
The data of Canadian colleagues who carried out a retrospective analysis of the annual survival rate of AMI patients in 109 hospitals in the province of Quebec, who received various ACE inhibitors, are extremely interesting. Interesting from the point of view that the real result of practitioners was assessed not in selectively selected patients, as is customary in test programs, but in the patient population of their region. The fate of 7,512 patients over the age of 65 was tracked. According to the results of the analysis, it was found that the most effective in reducing mortality within one year were ramipril and perindopril... In terms of their effectiveness, the remaining ACE inhibitors were ranked as follows: lisinopril\u003e enalapril\u003e quinapril\u003e fosinopril\u003e captopril.
The adjusted ratios of risk and confidence interval (confidence interval 95%) were, respectively: 0.98 (0.60-1.60); 1.28 (0.98-1.67); 1.47 (1.14-1.89); 1.58 (1.10-2.82); 1.56 (1.132.15). When ramipril was prescribed no earlier than 3-10 days from the onset of AMI, mortality during the first month decreased by 27%, within 15 months. - by 20%. That is, real practice has confirmed the validity of the conclusions of the two most significant programs - EUROPA on perindopril and HOPE by ramiprilu... Note that the data presented by the Canadians fit the results of two large studies - QUIT on quinapril and PEACE on trandolapril, in which, contrary to the expected, there was no improvement in the prognosis of life in people with a high risk of coronary heart disease who do not suffer from CHF and do not have left ventricular dysfunction.
In a theoretical discussion of two groups of neuromodulators - antagonists of receptors for angiotensin II and ACE inhibitors - the advantages of the former are undeniable. Confirmation of their pronounced organoprotective effect are, for example, the results of approbation of angiotensin II receptor antagonists in patients with renal dysfunction (RENAAL, LIFE) - the target organ of patients with arterial hypertension. In real practice, neither in patients with arterial hypertension with LVH (CATCH), nor in patients with CHF (ELITE II:; Val-HeFT :), the advantages of angiotensin II receptor antagonists over ACE inhibitors have not been proven. The words of the chief curator of the ONTARGET study, Canadian professor Salim Yusuf, expressed after a comparative analysis of thermisartan and ramipril at the 57th Annual Scientific Session of the American College of Cardiology in Chicago (2008), can be regarded as disappointing: “ Today, telmisartan is the only angiotensin II receptor antagonist drug with both cardio- and vasoprotective properties, the realization of which in a high-risk patient occurs regardless of the antihypertensive effect. In terms of protective effect, it is not inferior to ramipril».
Thus, at present, the most convincing data on the possibility of preventing the risk of chronic heart failure in patients with arterial hypertension are available from supporters of ACE inhibitors. In terms of reducing the risk of developing chronic heart failure, patients with arterial hypertension look preferable to others. perindopril and ramipril... The first turned out to be effective even in such a complex category of patients as patients with arterial hypertension of old age, that is, in people who have experienced many medicines, with the exception of calcium antagonists, has been unsuccessful.
Atroshchenko E.S., Atroshchenko I.E.
Republican Scientific and Practical Center "Cardiology" of the Ministry of Health of the Republic of Belarus; Belarusian medical Academy postgraduate education, Minsk.
Journal "Medical Panorama" No. 2, February 2009.
For citation:Podzolkov V.I., Osadchiy K.K. Cardiovascular Continuum: Can ACE Inhibitors Break the Vicious Circle? // RMJ. 2008. No. 17. S. 1102
Cardiovascular diseases (CVD) remain the leading cause of death in the modern world, claiming more than 17 million lives annually, mainly due to the development of fatal myocardial infarction (MI) and cerebral stroke.
The development of the most socially significant CVDs, which are based on the progression of atherosclerosis with the further occurrence of its complications, in the last 15 years is considered from the standpoint of the "cardiovascular continuum". This concept, first expressed by V. Dzau and E. Braunwald in 1991, has not only become generally accepted today, but in fact represents the cornerstone on which our understanding of the development of the most important CVDs is based. The cardiovascular continuum is a continuous chain of interrelated changes in the cardiovascular system from exposure to risk factors, through the gradual onset and progression of CVD to the development of terminal heart damage and death. Later, a "hypertensive cascade" of the cardiovascular continuum was proposed, in which the central role is played by arterial hypertension (AH) itself and hypertensive heart damage, leading in the final to the development of irreversible terminal changes, bypassing several stages of the classical continuum at once (Fig. 1).
A continuous chain of interrelated changes in the structure and function of several organs and systems of the body at once within the continuum suggests the presence of common pathophysiological processes, mechanisms of development and progression of organ damage. Basically, the whole variety of such mechanisms can be reduced to genetic, hemodynamic and neurohumoral factors. Among the latter, one of the central roles belongs to the activation of the renin-angiotensin-aldosterone system (RAAS), which can be traced at almost all stages of the cardiovascular continuum.
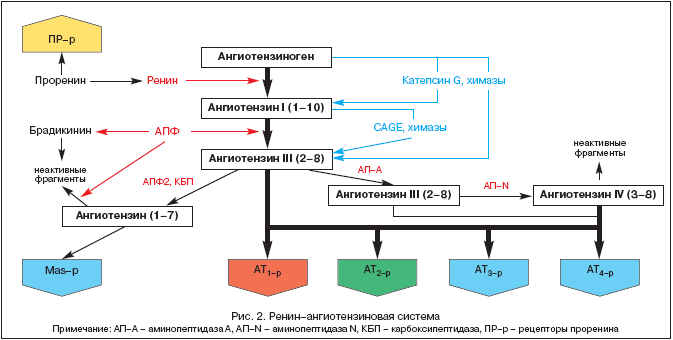
The history of the study of the RAAS dates back to 1898, when the Finnish physiologist Tigelstedt and his student Bergman isolated the first component of the RAAS, renin, from the kidney tissue, while not yet suspecting what role this fact would play in the development of pathophysiology, medicine and pharmacology in the 20th century. But only today, more than a hundred years later, the central role of RAAS and angiotensin II is becoming clearer not only in homeostatic regulation of blood pressure (BP), tissue perfusion, fluid and electrolyte balance, but also in a wide range of pathological processes. Modern ideas about the components of the RAAS are presented in Figure 2.
The RAAS is a unique regulatory system in which the active effector angiotensin II (Ang II) is produced in the extracellular space by sequential proteolytic cleavage of its precursors.
The predecessor of Ang II is angiotensinogen (Ang), a biologically inert globulin synthesized mainly in the liver (Ang mRNA expression was also detected in the kidneys, heart, brain, blood vessels, adrenal glands, ovaries, placenta, and adipose tissue). The concentration of Ang in the blood is practically stable. Renin, which is an acidic protease, is released into the blood by the juxtaglomerular apparatus of the kidneys in the form of a prohormone - prorenin, which accounts for up to 70-90% of all immunoreactive renin in the blood plasma. Prorenin receptors have recently been described and their role is being clarified. Renin can also be secreted by some other tissues (brain, heart, blood vessels). Renin acts on Ang and splits off several end fragments from it, which leads to the formation of angiotensin I (Ang I) or Ang- (1-10). It is this process that is rate-limiting in the entire cascade of formation of active metabolites of the RAAS. Ang I has biological activity and may act as a vasoconstrictor. Angiotensin-converting enzyme (ACE) is an exopeptidase localized on the membranes of various cells (endothelial, epithelial epithelial cells of the proximal renal tubules, neuro-epithelial cells) and in some amount in blood plasma. ACE cleaves off the terminal dipeptide from Ang I, converting Ang I into angiotensin II (Ang II) or Ang- (1-8) - the main effector of the RAAS. In addition, ACE metabolizes bradykinin and kallikrein to inactive metabolites.
Under the influence of endopeptidases found in the brain and kidneys, Ang III and Ang IV are formed from Ang II. The latter probably acts in conjunction with Ang II in the brain and increases blood pressure (BP).
Relatively recently, a new enzyme from the class of endopeptidases was isolated, called ACE2. Unlike ACE, it does not convert Ang I to Ang II and is not suppressed by ACE inhibitors (ACE inhibitors). Under the influence of ACE2, biologically inactive Ang- (1-9) is formed from Ang I, while Ang- (1-7) is formed under the action of tissue-specific endopeptidases and from Ang II with the participation of ACE2. Ang- (1-7) can be further metabolized with the participation of ACE to Ang- (1-5), the biological activity of which has not yet been clarified. The effects of Ang- (1-7) include vasodilation, increased diuresis and natriuresis, antitrophic action, which is realized through the stimulation of either specific receptors or MAS-p. Stimulation of the latter leads to an increase in the production of NO and prostacyclin. Today Ang- (1-7) is regarded as a natural ACE inhibitor. Apparently, Ang- (1-7) is one of the feedback components within the RAAS, exerting the opposite effect of Ang II. Thus, a certain balance is maintained between the pressor / trophic effects of Ang II and the depressor / atrophic effects of Ang II (1-7).
The main effector of the RAAS is Ang II, the action of which is realized through specific angiotensin receptors (AT-r). To the present moment, 4 subtypes of AT-p have been identified. AT is the most important 1-r, through the stimulation of which the majority of both physiological and pathophysiological effects of Ang II are realized (Table 1).
AT 1-r localized in blood vessels, heart, kidneys, adrenal glands, liver, brain and lungs. AT 2-r... are widely represented in the brain, kidneys and other tissues of the fetus, their number in the postnatal period decreases sharply. Nevertheless AT 2-r, in-vi-di-mo-mo, play a counter-regulatory role in relation to AT 1-r... (Table 1), which is confirmed during their blockade by the specific antagonist PD 123319. Functions of AT 3-r not studied, but AT stimulation 4-r Ang II, Ang III and Ang IV modulate the synthesis of the plasminogen activator inhibitor (PAI-1). Recently identified also specific receptors prorenin, their role is being specified. The experiment shows their role in the development of diabetic nephropathy.
Isolation of RAAS components from circulating blood and various tissues (heart, kidneys, brain, adrenal glands, adipose tissue, etc.) made it possible to form the concept of the presence of two links of the system - circulating RAAS and tissue RAAS. It is within the tissue RAAS (primarily of the kidneys and heart) that alternative pathways of Ang II formation without ACE involvement under the influence of chymases, cathessin G, and kallikrein-like enzymes have been identified.
Views on the place of RAAS in the regulation of functions human body in norm and pathology have been repeatedly revised. Today it is obvious that the RAAS is not only the most important regulatory system, but also plays a central role in a wide range of pathological processes in various tissues and organs of humans. Pronounced changes in the activity of the RAAS (both activation and suppression) were revealed in more than 30 nosologies and syndromes.
In experiments in vitro, on animal models in vivo and in human studies, the role of the RAAS (primarily the activation of its tissue link) in the development of essential and secondary hypertension, endothelial dysfunction, arterial remodeling and atherosclerosis, left ventricular hypertrophy (LVH), myocardial ischemia, cardiac remodeling after MI, CHF has been proven, diabetic and non-diabetic nephropathy, chronic renal failure (CRF) (Table 2).
In this way, the pathophysiological role of the RAAS is traced at all stages of the cardiovascular and renal continua .
Today in the arsenal of a doctor there are three groups of drugs that can block the activity of the RAAS - ACE inhibitors, AT blockers 1 -Angiotensin receptor (ARB), direct renin inhibitor (aleskiren).
The first drugs blocking the RAAS were ACE inhibitors, the development of which began in the 60s of the XX century, and the first non-peptide ACE inhibitors captopril was synthesized in 1975. To date, ACE inhibitors are the most important class of drugs used in cardiology, and are included in the group so called life-saving drugs due to their proven ability to improve prognosis in a variety of cardiovascular and renal diseases.
The mechanism of action of an ACE inhibitor is the competitive suppression of ACE, which, on the one hand, leads to a decrease in the formation of Ang II, the main effector of the RAAS, and on the other hand, reduces the degradation of bradykinin, kallikrein, substance R. This causes pharmacological effects ACE inhibitors : reduction of vascular resistance, improvement of endothelial function, antiproliferative effect, effect on the blood coagulation system, improvement of renal function.
The mechanism of action and the main pharmacological effects are the same for the entire class of ACE inhibitors. However, the choice of a specific drug from the ACE inhibitor group for the treatment of a specific patient may be important. ACE inhibitors are a heterogeneous group of drugs that differ from each other both in chemical structure, features of pharmacokinetics and pharmacodynamics, and in the presence of an evidence base for use for various indications. It is important to understand that although the ability of an ACE inhibitor to lower blood pressure and slow the progression of CHF are regarded as class-effects, many organ-protective effects of individual ACE inhibitors cannot be transferred from the standpoint of evidence-based medicine to the entire class of drugs.
ACE inhibitors differ in their chemical structure (the presence of a sulfhydryl group, etc.), metabolic characteristics (the presence of the effect of the first passage through the liver), the characteristics of excretion from the body (only by the kidneys or kidneys together with the liver), tissue specificity (the ability to block tissue RAAS) and duration actions (Table 3).
One of the most studied a wide range indications of an ACE inhibitor is ramipril (Tritace ® ). The drug is characterized by high lipophilicity (superior to enalapril by almost 20 times), tissue specificity (superior to enalapril by 3-10 times, depending on tissue), a long half-life, which allows it to be used once a day. It is especially important to note that the evidence base for the use of ramipril in CVD, based on the results of RCTs with hard endpoints, is by far the largest among all ACE inhibitors.
The antihypertensive efficacy and safety of ramipril was evaluated in a large open-label study CARE conducted in a real clinical practice... The trial included 11100 patients with grade I-II hypertension, the effectiveness of treatment was assessed in 8261 patients. Ramipril was prescribed as monotherapy at a dose of 2.5 to 10 mg / day. After 8 weeks of treatment, there was a significant decrease in both SBP and DBP by an average of 13%, and this effect was also observed in the group of patients with isolated systolic hypertension (ISAG). The response rate to treatment (reaching target blood pressure below 140 and 90 mm Hg or decrease in DBP\u003e 10 mm Hg, or decrease in SBP\u003e 20 mm Hg with ISAH) was more than 85 in the systolic-diastolic hypertension group. %, and in the ISAG group more than 70%. The number of side effects during therapy, estimated in 11,100 patients, was low, and the cough rate did not exceed 3%.
Numerous studies have shown that ACE inhibitors are capable of causing regression of LVH and this effect is due not only to a decrease in blood pressure, but also to the blockade of the RAAS itself.
Meta-analyzes of RCTs that investigated the potential for different classes of antihypertensive drugs to induce regression of LVH also revealed the advantages of ACE inhibitors over other drugs.
The ability of ramipril to reduce the severity of LVH was studied in a double-blind, placebo-controlled RCT HYCAR ... In the course of the study, 115 patients with hypertension were prescribed either ramipril in doses of 1.25 mg / day. and 5 mg / day, or placebo. After 6 months, LV myocardial weight significantly increased in the placebo group and significantly decreased in the ramipril groups. A greater decrease was observed in the group with a dose of ramipril 5 mg / day. ... In an open multicenter RCT with a blind endpoint RACE in 193 patients with hypertension I-II stage. compared the effects of ramipril and atenolol on blood pressure levels and LV myocardial mass assessed by echocardiography. Ramipril was prescribed at a dose of 2.5 mg / day, atenolol at a dose of 50 mg / day. with the subsequent possibility of doubling the dose after 2 weeks. The duration of the test was 6 months. As a result, it was noted that both ramipril and atenolol significantly reduced both SBP and DBP, and equally. However, a significant decrease in the LV myocardial mass index was observed only in the ramipril group.
A large RCT has become an important milestone in the study of the possibilities of ACE inhibitors in the prevention of complications in high-risk patients HOPE (Heart Outcome Prevention Evaluation). The aim of the study was to assess the possibility of reducing morbidity and mortality from CVD in high-risk patients under the influence of two treatment strategies: ACE inhibitors ramipril and vitamin E. This double-blind, placebo-controlled study with a factorial design enrolled 9541 patients at high risk of cardiovascular complications due to age (\u003e 55 years), the presence of cardiovascular diseases, or diabetes mellitus in combination with vascular disease or risk factors (hypertension, smoking, dyslipidemia). The peculiarities of the population of patients included in the study were the absence of LV dysfunction and CHF, a low mean baseline blood pressure (139 and 79 mm Hg), although almost half of those included in the study had AH, the use of other medicinal drugs that could affect treatment outcomes. So, 76% of patients received antiplatelet drugs (mainly acetylsalicylic acid (ASA)), 45% - calcium antagonists, 40% - β-adreno-blockers, 30% - lipid-lowering drugs, 15% - diuretics. In the course of the study, the frequency of the use of lipid-lowering drugs, β-blockers and diuretics increased, and the frequency of calcium antagonists decreased by 5%. The initially low values \u200b\u200bof blood pressure in the study population are explained precisely by the widespread use of antihypertensive drugs. Ramipril was prescribed starting at a dose of 2.5 mg / day, followed by titration up to 10 mg / day. The maximum dose by the end of the first year of the study was received by 82% of patients, and by the end of the study (4.5 years) - 65% of patients. The primary endpoint of the study was a combination of cardiovascular death, nonfatal MI, and nonfatal stroke.
The HOPE study was stopped early (six months earlier) due to the clear advantages of ramipril over vitamin E. The effectiveness of the latter did not differ from placebo. The rate of reaching the primary endpoint in the ramipril group was 14% compared with 17.8% in the placebo group, which corresponds to a 22% reduction in the relative risk (p<0,001). Относительный риск развития отдельных компонентов первичной конечной точки также снизился: инсульта на 32%, ИМ на 20%, сердечно-сосудистой смерти на 26%. Применение рамиприла обеспечило также достоверное снижение риска развития ХСН (на 23%) и проведения процедур реваскуляризации (на 15%). Важнейшим результатом исследования НОРЕ стало снижение под влиянием рамиприла общей смертности на 16% (р=0,005), причем кривые Капла-на-Майе-ра разошлись уже к первому году и продолжали расходиться до конца исследования.
The results of the study did not depend on the use of other drugs and were significant for various subgroups (with diabetes, hypertension, previous vascular lesions, men and women).
With ramipril, the incidence of new cases of diabetes was 33% lower than with placebo.
An important finding from the HOPE study was that the decline in endpoint occurrence was much more pronounced than expected from a BP decline. That is, the protective effects of ramipril clearly extended beyond its antihypertensive action. This made it possible to assume that ramipril actively influenced the processes of vascular remodeling and atherogenesis.
The ability of an ACE inhibitor to inhibit the development of atherosclerosis has been demonstrated in animals. However, human studies have produced conflicting results. Among all ACE inhibitors tested for the possibility of inhibition of atherogenesis in vivo, the largest evidence base has been accumulated for ramipril and perindopril. As part of the test NORE under investigation SECURE , which evaluated the ability of ramipril to slow the progression of atherosclerosis in 753 patients. With the use of a high dose of ramipril (10 mg / day), there was a 37% slowdown in the progression of atherosclerosis in the carotid artery compared with placebo, assessed by an increase in the thickness of the intima / media complex (IMC). In the low-dose ramipril group (2.5 mg / day), the IMC thickness was also smaller than in the placebo group, but the differences were not significant. Thus, the antiatherogenic effect of ramipril can be considered proven, but it should be considered as dose-dependent.
The anti-atherogenic effect of ramipril, identified in the SECURE study, apparently explains in many ways the drug's efficacy in secondary prevention of CVD, convincingly demonstrated in the HOPE study.
The continuation of the NORE study was the project NORE-TOO to assess whether ramipril continues to be able to reduce adverse cardiovascular events and new cases of diabetes over time in high-risk patients. The study included 4528 patients from the HOPE study who either continued to take ramipril 10 mg / day. in the open mode, or switched to ramipril after taking a placebo. By the end of the follow-up period (2.6 years), there was a further significant decrease in the relative risk of the primary endpoint by 17%, MI by 19%, revascularization procedures by 16%, and new cases of diabetes by 34%. Reductions in the relative risk of adverse events have been observed in various patient subgroups, including low, medium, and high risk subgroups. Thus, it has been proven that the protective effects of ramipril not only persist over time, but their severity is significantly higher than was shown in the HOPE study.
The use of ramipril for MI complicated by the development of heart failure has been studied in a large, double-blind, placebo-controlled RCT AIRE ... The trial included 2006 patients with confirmed MI and symptoms of heart failure. Ramipril was prescribed at a dose of 5 mg / day, starting from 3-10 days of illness, followed by titration up to 10 mg / day. within 2 days. The primary endpoint was overall mortality, the secondary endpoint was adverse cardiovascular events (death, re-heart attack, stroke, progression of heart failure). The duration of the study was 15 months on average. (minimum 6 months). 59% of patients in the ramipril group underwent thrombolysis, 77% took ASA, 25% - β-blockers, 56% - ni-waste. The use of ramipril provided a significant reduction in overall mortality by 27%, which became apparent after 30 days of treatment. The relative risk of secondary endpoints was significantly reduced by 19%. At the same time, the survival curves continued to diverge throughout the study (up to 30 months). The effect of ramipril persisted in various subgroups of patients (men and women, with and without hypertension, and others). The drug withdrawal rate was not significantly different from the placebo withdrawal rate.
Continuation AIRE there was a study AIREX , the purpose of which was to evaluate the effectiveness of long-term (5 years) therapy with ramipril in patients with MI with symptoms of heart failure. The trial included 603 patients from the AIRE study who continued to receive either ramipril or placebo. The duration of treatment was 59 months on average. (minimum 42 months). As a result, by the 59th month, the absolute survival rate was 11.4% higher in the ramipril group, which corresponds to a significant decrease in the relative risk of death by 36%. The average increase in life expectancy in the ramipril group was 1.45 g. As a result, not only was the high efficacy of the drug in this group of patients and its preservation over time confirmed again. It was also concluded that "treatment with ramipril 5 mg twice daily after acute MI, once started, should continue indefinitely."
The beneficial effect of ramipril on the survival of elderly patients with myocardial infarction was shown in a Canadian retrospective study, which included 7512 patients over 65 years old who received various ACE inhibitors after discharge from the hospital. As a result, ramipril significantly surpassed enalapril, fosinopril, captopril, quinapril and lisinopril in terms of its effect on survival during the first year.
Interesting comparative data were obtained when analyzing the outcomes of patients included in the registry MITRA PLUS ... Among 14608 patients with ST-segment elevation MI, 4.7% received ramipril, 39.0% - other ACE inhibitors, 56.3% - did not receive an ACE inhibitor. Compared with the absence of ACE inhibitors and, which is especially important, compared with other ACE inhibitors, treatment with ramipril provided significantly lower rates of hospital mortality and the incidence of adverse cardiovascular and cerebral events. However, there were no differences in the incidence of heart failure between ACE inhibitors.
Interesting data from a double-blind, placebo-controlled study DIAB-HYCAR , in which the effect of low doses of ramipril (1.25 mg / day) on the incidence of cardiovascular and renal complications in 4912 patients with type 2 diabetes and nephropathy manifested by microalbuminuria or proteinuria was evaluated. The use of the drug at such a low dose contributed to a slight decrease in blood pressure and a decrease in protein excretion in the urine, but did not lead to a significant decrease in either cardiovascular or renal endpoints. This result once again emphasizes that the beneficial effects of ramipril are realized when the appropriate doses are used - 10 mg / day.
The largest comparative RCT recently completed ONTARGET , which compared the possibilities of preventing complications in patients with CVD or diabetes mellitus without heart failure using three regimens of therapy: ACE inhibitors, ARBs, and a combination of ACE inhibitors + ARBs. The study included 25620 patients with coronary artery disease, peripheral vascular disease, cerebrovascular disease or diabetes mellitus. At baseline, 89% of patients had CVD, 69% had hypertension, and 38% had diabetes. When included in the study, 80.9% of patients took antiplatelet agents, 61.6% - statins, 56.9% - β - adrenergic blockers, 28.0% - diuretics. Patients were randomized into three groups: taking ramipril at a dose of 10 mg / day. (n \u003d 8502) taking telmisartan 80 mg / day. (n \u003d 8542) and taking a combination of ramipril with telmisartan (n \u003d 8502). The observation period was 56 months.
As a result, 16.5% of patients in the ramipril group, 16.7% in the telmisartan group and 16.3% in the combination treatment group achieved the primary combined endpoint, which included mortality from complications of CVD, MI, stroke, or hospitalization for heart failure. That is, there was no difference between ramipril monotherapy, telmisartan monotherapy, and combination therapy with both drugs. The incidence of individual adverse outcomes included in the composite indicator and overall mortality did not differ significantly either. At the same time, deterioration of renal function was more often observed in the combination therapy group: the relative risk of developing chronic renal failure was 1.33 (p<0,001) .
Thus, this largest comparative study did not reveal the advantages of using ARBs over traditional therapy with ACE inhibitors in patients with CVD and diabetes mellitus, with the exception of a slightly lower incidence of angioedema. In fact, telmisartan at a dose of 80 mg / day. provided 94% of the effectiveness of ramipril at the 10 mg / day dose established in the HOPE study. These data are consistent with the results of the RCT VALIANT, in which the effect of valsartan also did not exceed the effect of captopril.
All this allowed J.McMurray in an editorial New England Journal of Medicineto express the opinion that since ARBs are not superior to traditional ACE inhibitors in terms of effectiveness, but are significantly more expensive, their area of \u200b\u200bapplication mainly comes down to cases of intolerance to ACE inhibitors due to cough.
The results of the ONTARGET study are of great scientific importance, not only in practical terms. They once again force us to pay attention to the alleged role of bradykinin in ensuring the clinical efficacy of drugs that block RAAS. And although ACE inhibitors do not completely block the formation of Ang II, unlike ARBs, they reduce the degradation of bradykinin to inactive metabolites.
Thus, the available RCT results with ramipril indicate that the drug provides a positive effect on endpoints, including overall mortality, in various CVDs. In fact, this makes it possible to provide organoprotection at various stages of the cardiovascular (including the hypertensive cascade) continuum, ranging from exposure to risk factors (primarily hypertension and diabetes) and ending with terminal organ damage (CHF). At the same time, it is necessary to emphasize the importance of choosing the correct dosage for the drug and the need for long-term, often lifelong treatment.


Literature
1. Ezzati M, Hoorn SV, Rodgers A, et al. 2003. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet 362: 271-80.
2. Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J. 1991 Apr; 121 (4 Pt 1): 1244-63.
3. Victor J. Dzau, Elliott M. Antman, Henry R. Black et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006 Dec 19; 114 (25): 2850-70.
4. V. I. Podzolkov, V. A. Bulatov. Myocardium. Nephron. A look through the prism of the evolution of arterial hypertension. RMJ 2008, 16 (11): 1517-1523.
5. Morgan L, Broughton PF, Kalsheker N. Angiotensinogen: molecular biology, biochemistry and physiology. Int J Biochem Cell Biol. 1996; 28: 1211-22.
6. Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003; 24: 261-71.
7. Reudelhuber TL. The renin-angiotensin system: peptides and enzymes beyond angiotensin II. Curr Opin Nephrol Hypertens. 2005; 14: 155-59.
8. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000 Sep 1; 87 (5): E1-9.
9. Tallant EA, Ferrario CM, Gallagher PE. Angiotensin- (1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 2005; 289: H1560-H1566.
10. Stanton A. Therapeutic potential of renin inhibitors in the management of cardiovascular disorders. Am J Cardiovasc Drugs. 2003; 3: 389-94.
11. Ichihara A, Hayashi M, Kaneshiro Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004; 114: 1128-35.
12. Phillips MI. Tissue renin-angiotensin systems. In: Izzo JL, Black HR, ed. Hypertension Primer: The Essentials of High Blood Pressure. 2nd ed. Baltimore, MD: Lippincott William & Wilkins; 1999: 23-24.
13. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006; 86: 747-803.
14. Lopez-Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J. 2004 Aug; 25 (16): 1454-70.
15. Kaplan NM. The CARE Study: a postmarketing evaluation of ramipril in 11,100 patients. The Clinical Altace Real-World Efficacy (CARE) Investigators. Clin Ther. 1996 Jul-Aug; 18 (4): 658-70.
16. Dahlof B, Pennert K, Hansson L. Reversal of left ventricular hypertrophy in hypertensive patients. A metaanalysis of 109 treatment studies. Am J Hypertens. 1992 Feb; 5 (2): 95-110.
17. Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double-blind studies. JAMA. 1996 May 15; 275 (19): 1507-13.
18. Lievre M, Gueret P, Gayet C, et al. Remission of left ventricular hypertrophy with ramipril independently of blood pressure changes: the HYCAR study (cardiac hypertrophy and ramipril)] Arch Mal Coeur Vaiss. 1995 Feb; 88 Spec # 2: 35-42.
19. Agabiti-Rosei E, Ambrosioni E, Dal Palu C, Muiesan ML, Zanchetti A. ACE inhibitor ramipril is more effective than the beta-blocker atenolol in reducing left ventricular mass in hypertension. Results of the RACE (ramipril cardioprotective evaluation) study on behalf of the RACE study group. J Hypertens. 1995 Nov; 13 (11): 1325-34.
20. The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on death from cardiovascular causes, myocardial infarction and stroke in high-risk patients.New Engl J Med 2000; 342: 145-153.
21. Pitt B. Potential role of angiotensin-converting enzyme inhibitors in the treatment of atherosclerosis. Eur Heart J 1995; 16: 49-54.
22. Schoelkens BA, Landgraf W. ACE inhibition and atherosclerosis. Can J Physiol Pharmacol 2002; 80: 354-9.
23. Lonn E, Yusuf S, Dzavik V, et al. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation. 2001 Feb 20; 103 (7): 919-25.
24. Bosch J, Lonn E, Pogue J, Arnold JM, Dagenais GR, Yusuf S; HOPE / HOPE-TOO Study Investigators. Long-term effects of ramipril on cardiovascular events and on diabetes: results of the HOPE study extension. Circulation. 2005 Aug 30; 112 (9): 1339-46.
25. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993 Oct 2; 342 (8875): 821-8.
26. Hall AS, Murray GD, Ball SG. Follow-up study of patients randomly allocated ramipril or placebo for heart failure after acute myocardial infarction: AIRE Extension (AIREX) Study. Acute Infarction Ramipril Efficacy. Lancet. 1997 May 24; 349 (9064): 1493-7.
27. Pilote L, Abrahamowicz M, Rodrigues E, Eisenberg MJ, Rahme E. Mortality rates in elderly patients who take different angiotensin-converting enzyme inhibitors after acute myocardial infarction: a class effect? Ann Intern Med. 2004 Jul 20; 141 (2): 102-12.
28. Wienbergen H, Schiele R, Gitt AK, et al. Impact of ramipril versus other angiotensin-converting enzyme inhibitors on outcome of unselected patients with ST-elevation acute myocardial infarction. Am J Cardiol. 2002 Nov 15; 90 (10): 1045-9.
29. Michel Marre, Michel Lievre, Gilles Chatellier, et al. on behalf of the DIABHYCAR Study Investigators. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomized, double blind, placebo controlled trial (the DIAB-HYCAR study). BMJ 2004; 328; 495.
30. The ONTARGET Investigators. Telmisartan, ramipril or both in patients at a high risk for vascular events. N Eng J Med 2008; 358: 1547-1559
31. Pfeffer M, McMurray J, Velazquez E, et al. Valsartan, captopril or both in myocardial infarction complicated by heart failure, left ventricle dysfunction. N Eng J Med 2003; 349: 1893-1906
32. McMurray J. ACE-inhibitors in cardiovascular disease - unbeatable? N Eng J Med 2008; 358: 1615-1616
GBOU VPO SOGMA
Ministry of Health of the Russian Federation
Department of Internal Medicine № 4.
Head department d. m. n. PROFESSOR ASTAKHOVA Z.T.
Methodical instructions for conducting a practical lesson
with 6th year students of the Faculty of General Medicine on the topic:
DIFFERENTIAL DIAGNOSTICS OF MAIN SYNDROMES IN CHRONIC HEART FAILURE. TREATMENT OF CHRONIC HEART FAILURE.
(lesson duration 8 hours, first lesson)
Vladikavkaz - 2014 - 2015 academic year year.
Methodical instructions for conducting a practical lesson with 6th year students of the Faculty of General Medicine on the topic:
DIFFERENTIAL DIAGNOSTICS OF BASIC
SYNDROMES IN CHF. CHF TREATMENT.
The purpose of the lesson:
define the concept and classification of chronic heart failure
to consider the general mechanisms of the onset of chronic heart failure and the main differential diagnostic signs of various types of chronic heart failure
study modern methods for verifying chronic heart failure and determining its stage and severity.
Motivation for the relevance of the topic:
CHF is the most common reason for hospitalization of patients in cardiological hospitals (almost every second patient - 49%)
CHF is diagnosed in 92% of patients.
The one-year mortality rate of patients with clinically severe CHF reaches 26-29%, i.e. in one year in Russia, from 880 to 986 thousand patients with CHF die.
In addition, the successes of modern medicine determine the "aging" of the population. In patients over 65 years of age, the incidence of CHF reaches 10% and is the main cause of disability and mortality, significantly worsens the quality of life and requires significant financial costs.
To improve the quality of life of ballroom patients with CHF, early diagnosis and prevention of CHF is necessary, and in the presence of clinical manifestations, constant adequate therapy.
Determination of the level of training of students:
The second level of knowledge: control methods - written survey (20 min)
(students should know:
The main indicators of hemodynamics
1. The final systolic volume - CSR.
2. End diastolic volume - EDV.
3. CV of blood \u003d EDV - CSR (EDV (130 ml) - CSR (60 ml) \u003d CVR (70 ml).
4. PV \u003d VO / EDV (normal \u003d 0.6 or 60%).
5. Cardiac output \u003d SV x HR (5 l / min).
6. SI \u003d SV / S (3 l / min / square meter).
7. Average BP \u003d DBP + 1/3 pulse BP.
8. OPSS (in a large circle) \u003d average blood pressure / SV (normal from 700 to 1600 (dynes cm) -5.
9. Contractility - the degree and speed of shortening of myocardial waves (in the clinic they are judged by EF and the speed of circulatory shortening of the fibers of the left ventricle).
the degree of distension of the heart during diastole. Depends on the amount of venous inflow to the heart and myocardial extensibility (judged by EDV).11. Afterload - the degree of myocardial tension during systole, ie. force of resistance to shortening of myocardial fibers; depends on the pressure and volume of the cavity, the thickness of the LV wall at the time of the opening of the aortic valve.
CHF (definition of GFCF)
A disease with a complex of characteristic symptoms (shortness of breath, fatigue, decreased physical activity, edema, etc.), which are associated with inadequate perfusion of organs and tissues at rest or during exertion and often with fluid retention in the body. The root cause is a deterioration in the heart's ability to fill or empty due to myocardial damage, as well as an imbalance of the vasoconstrictor and vasodilating neurohumoral systems.
Classification of the reasons for the development of HNK (after N.M. Mukharlyamov, 1987)
I. Damage to the heart muscle(myocardial insufficiency):
and. primary (myocarditis, DCM);
b. secondary (atherosclerotic and postinfarction cardiosclerosis, hypo- or hyperthyroidism, heart damage in systemic connective tissue diseases, toxicoallergic myocardial damage).
II. Hemodynamic overload of the heart muscle:
and. pressure (stenosis of the mitral, tricuspid valves, orifice of the aorta and pulmonary artery, hypertension of the pulmonary or systemic circulation);
b. volume (insufficiency of heart valves, intracardiac shunts);
at. combined (complex heart defects, a combination of pathological processes leading to pressure or volume overload).
III. Violation of diastolic ventricular filling:
and. Adhesive pericarditis
b. Restrictive cardiomyopathies.
The most common cause of CHF is ischemic (coronary) heart disease (CHD) - 60%. In second place are valvular heart defects (14%), followed by dilated cardiomyopathy (11%). A single cardiovascular continuum, or the continuous development of cardiovascular diseases - from risk factors to the death of a patient.
CARDIOVASCULAR CONTINUUM
Risk factors, the main of which must be recognized as hypertension, dyslipidemia and diabetes, provoke the development of coronary artery disease, which can be complicated by AMI. As a result, contractile elements are lost, LV remodeling develops, then CHF and the patient dies. The second scenario after AMI and with developed CHF is arrhythmic, sudden death of the patient. Shorter connections are also possible, for example, from hypertension to LV hypertrophy, its dysfunction and then to CHF. From chronic ischemic heart disease to myocardial hibernation, the same LV dysfunction and decompensation.
CHF classification.
Classification of V.Kh. Vasilenko and N.D. Strazhesko (1935):
STAGE I - initial, latent circulatory failure, manifested only during physical exertion (shortness of breath, palpitations, excessive fatigue). At rest, these phenomena disappear. Hemodynamics is not impaired.
STAGE II - severe long-term circulatory failure, hemodynamic disturbances (stagnation in the pulmonary and systemic circulation) are expressed at rest.
period A - signs of circulatory failure at rest are moderately expressed. Hemodynamic disturbances in only one of the parts of the cardiovascular system (in a large orsmall circle of blood circulation).
period B - the end of a long stage, pronounced hemodynamic disturbances, in which the entire cardiovascular system is involved ( andlarge, andsmall circle of blood circulation).
STAGE III - final, dystrophic with severe hemodynamic disturbances, persistent metabolic changes and irreversible changes in the structure of organs and tissues.
period A - characterized by pronounced signs of CHF at rest, the presence of common phenomena of decompensation in two circles of blood circulation and hemodynamic disorders. However, with active complex therapy, it is possible to significantly eliminate the severity of stagnation, stabilize hemodynamics and gradually restore the functions of vital organs.
period B - irreversible changes.
Functional classification of CHF (1964):
I FC - the patient does not experience restrictions in physical activity. Normal exercise does not cause weakness (lightheadedness), palpitations, shortness of breath, or anginal pain.
II FC - moderate limitation of physical activity. The patient feels comfortable at rest, but doing normal physical activity causes weakness (lightheadedness), palpitations, shortness of breath, or anginal pain.
III FC - pronounced limitation of physical activity. The patient feels at rest, but less than usual physical activity leads to the development of weakness (lightheadedness), palpitations, shortness of breath or anginal pain.
IV FC - inability to perform any loads without the appearance of discomfort. Symptoms of heart failure or angina syndrome may present
Thus, the functional classification of CHF reflects the ability of patients to perform physical activities and outlines the degree of changes in the functional reserves of the body, which is especially important when assessing the dynamics of the state of patients.
The main reasons for the development of CHF
(N.M. Mukharlyamov, 1978; B.A.Sidorenko, D.V. Preobrazhensky, 1995):
1.Myocardial damage (mainly systolic insufficiency):
Myocarditis;
Dilated cardiomyopathy;
· Myocardial dystrophy;
Ischemic heart disease (atherosclerotic and postinfarction cardiosclerosis)
2. Pressure overload (systolic);
Arterial hypertension (systemic and pulmonary);
Aortic stenosis (subvalvular, valvular, supravalvular)
Narrowing of the mouth of the pulmonary artery
3.Volume overload (diastolic):
· Aortic or mitral regurgitation;
· Defect of the interventricular septum;
Open arterial duct
4. Combined overload.
5. Violation of diastolic ventricular filling (diastolic insufficiency):
Hypertrophic cardiomyopathy;
· Restrictive cardiomyopathy;
Hypertensive heart (in the absence of LV dilatation);
· Isolated mitral stenosis;
Pericarditis (exudative and constrictive).
6.Conditions with high cardiac output:
Thyrotoxicosis;
· Anemia;
· Massive obesity;
· cirrhosis of the liver.
Principles of CHF diagnosis
The presence of characteristic clinical symptoms of HF (mainly shortness of breath, fatigue, limited physical activity, and ankle edema)
Objective evidence that these symptoms are associated with damage to the heart, and not any other organs (lungs, anemia, kidney failure)
In doubtful cases, confirms the diagnosis of a positive response to treatment
For citation:Podzolkov V.I., Osadchiy K.K. Cardiovascular Continuum: Can ACE Inhibitors Break the Vicious Circle? // RMJ. 2008. No. 17. S. 1102
Cardiovascular diseases (CVD) remain the leading cause of death in the modern world, claiming more than 17 million lives annually, mainly due to the development of fatal myocardial infarction (MI) and cerebral stroke.
The development of the most socially significant CVDs, which are based on the progression of atherosclerosis with the further occurrence of its complications, in the last 15 years is considered from the standpoint of the "cardiovascular continuum". This concept, first expressed by V. Dzau and E. Braunwald in 1991, has not only become generally accepted today, but in fact represents the cornerstone on which our understanding of the development of the most important CVDs is based. The cardiovascular continuum is a continuous chain of interrelated changes in the cardiovascular system from exposure to risk factors, through the gradual onset and progression of CVD to the development of terminal heart damage and death. Later, a "hypertensive cascade" of the cardiovascular continuum was proposed, in which the central role is played by arterial hypertension (AH) itself and hypertensive heart damage, leading in the final to the development of irreversible terminal changes, bypassing several stages of the classical continuum at once (Fig. 1).
A continuous chain of interrelated changes in the structure and function of several organs and systems of the body at once within the continuum suggests the presence of common pathophysiological processes, mechanisms of development and progression of organ damage. Basically, the whole variety of such mechanisms can be reduced to genetic, hemodynamic and neurohumoral factors. Among the latter, one of the central roles belongs to the activation of the renin-angiotensin-aldosterone system (RAAS), which can be traced at almost all stages of the cardiovascular continuum.
The history of the study of the RAAS dates back to 1898, when the Finnish physiologist Tigelstedt and his student Bergman isolated the first component of the RAAS, renin, from the kidney tissue, while not yet suspecting what role this fact would play in the development of pathophysiology, medicine and pharmacology in the 20th century. But only today, more than a hundred years later, the central role of RAAS and angiotensin II is becoming clearer not only in homeostatic regulation of blood pressure (BP), tissue perfusion, fluid and electrolyte balance, but also in a wide range of pathological processes. Modern ideas about the components of the RAAS are presented in Figure 2.
The RAAS is a unique regulatory system in which the active effector angiotensin II (Ang II) is produced in the extracellular space by sequential proteolytic cleavage of its precursors.
The predecessor of Ang II is angiotensinogen (Ang), a biologically inert globulin synthesized mainly in the liver (Ang mRNA expression was also detected in the kidneys, heart, brain, blood vessels, adrenal glands, ovaries, placenta, and adipose tissue). The concentration of Ang in the blood is practically stable. Renin, which is an acidic protease, is released into the blood by the juxtaglomerular apparatus of the kidneys in the form of a prohormone - prorenin, which accounts for up to 70-90% of all immunoreactive renin in the blood plasma. Prorenin receptors have recently been described and their role is being clarified. Renin can also be secreted by some other tissues (brain, heart, blood vessels). Renin acts on Ang and splits off several end fragments from it, which leads to the formation of angiotensin I (Ang I) or Ang- (1-10). It is this process that is rate-limiting in the entire cascade of formation of active metabolites of the RAAS. Ang I has biological activity and may act as a vasoconstrictor. Angiotensin-converting enzyme (ACE) is an exopeptidase localized on the membranes of various cells (endothelial, epithelial epithelial cells of the proximal renal tubules, neuro-epithelial cells) and in some amount in blood plasma. ACE cleaves off the terminal dipeptide from Ang I, converting Ang I into angiotensin II (Ang II) or Ang- (1-8) - the main effector of the RAAS. In addition, ACE metabolizes bradykinin and kallikrein to inactive metabolites.
Under the influence of endopeptidases found in the brain and kidneys, Ang III and Ang IV are formed from Ang II. The latter probably acts in conjunction with Ang II in the brain and increases blood pressure (BP).
Relatively recently, a new enzyme from the class of endopeptidases was isolated, called ACE2. Unlike ACE, it does not convert Ang I to Ang II and is not suppressed by ACE inhibitors (ACE inhibitors). Under the influence of ACE2, biologically inactive Ang- (1-9) is formed from Ang I, while Ang- (1-7) is formed under the action of tissue-specific endopeptidases and from Ang II with the participation of ACE2. Ang- (1-7) can be further metabolized with the participation of ACE to Ang- (1-5), the biological activity of which has not yet been clarified. The effects of Ang- (1-7) include vasodilation, increased diuresis and natriuresis, antitrophic action, which is realized through the stimulation of either specific receptors or MAS-p. Stimulation of the latter leads to an increase in the production of NO and prostacyclin. Today Ang- (1-7) is regarded as a natural ACE inhibitor. Apparently, Ang- (1-7) is one of the feedback components within the RAAS, exerting the opposite effect of Ang II. Thus, a certain balance is maintained between the pressor / trophic effects of Ang II and the depressor / atrophic effects of Ang II (1-7).
The main effector of the RAAS is Ang II, the action of which is realized through specific angiotensin receptors (AT-r). To the present moment, 4 subtypes of AT-p have been identified. AT is the most important 1-r, through the stimulation of which the majority of both physiological and pathophysiological effects of Ang II are realized (Table 1).
AT 1-r localized in blood vessels, heart, kidneys, adrenal glands, liver, brain and lungs. AT 2-r... are widely represented in the brain, kidneys and other tissues of the fetus, their number in the postnatal period decreases sharply. Nevertheless AT 2-r, in-vi-di-mo-mo, play a counter-regulatory role in relation to AT 1-r... (Table 1), which is confirmed during their blockade by the specific antagonist PD 123319. Functions of AT 3-r not studied, but AT stimulation 4-r Ang II, Ang III and Ang IV modulate the synthesis of the plasminogen activator inhibitor (PAI-1). Recently, specific prorenin receptors have also been identified, and their role is being clarified. The experiment shows their role in the development of diabetic nephropathy.
Isolation of RAAS components from circulating blood and various tissues (heart, kidneys, brain, adrenal glands, adipose tissue, etc.) made it possible to form the concept of the presence of two links of the system - circulating RAAS and tissue RAAS. It is within the tissue RAAS (primarily of the kidneys and heart) that alternative pathways of Ang II formation without ACE involvement under the influence of chymases, cathessin G, and kallikrein-like enzymes have been identified.
The views on the place of the RAAS in the regulation of the functions of the human body in health and disease have been repeatedly revised. Today it is obvious that the RAAS is not only the most important regulatory system, but also plays a central role in a wide range of pathological processes in various tissues and organs of humans. Pronounced changes in the activity of the RAAS (both activation and suppression) were revealed in more than 30 nosologies and syndromes.
In experiments in vitro, on animal models in vivo and in human studies, the role of the RAAS (primarily the activation of its tissue link) in the development of essential and secondary hypertension, endothelial dysfunction, arterial remodeling and atherosclerosis, left ventricular hypertrophy (LVH), myocardial ischemia, cardiac remodeling after MI, CHF has been proven, diabetic and non-diabetic nephropathy, chronic renal failure (CRF) (Table 2).
In this way, the pathophysiological role of the RAAS is traced at all stages of the cardiovascular and renal continua .
Today in the arsenal of a doctor there are three groups of drugs that can block the activity of the RAAS - ACE inhibitors, AT blockers 1 -Angiotensin receptor (ARB), direct renin inhibitor (aleskiren).
The first drugs blocking the RAAS were ACE inhibitors, the development of which began in the 60s of the XX century, and the first non-peptide ACE inhibitors captopril was synthesized in 1975. To date, ACE inhibitors are the most important class of drugs used in cardiology, and are included in the group so called life-saving drugs due to their proven ability to improve prognosis in a variety of cardiovascular and renal diseases.
The mechanism of action of an ACE inhibitor is the competitive suppression of ACE, which, on the one hand, leads to a decrease in the formation of Ang II, the main effector of the RAAS, and on the other hand, reduces the degradation of bradykinin, kallikrein, substance R. This causes pharmacological effects of ACE inhibitors : reduction of vascular resistance, improvement of endothelial function, antiproliferative effect, effect on the blood coagulation system, improvement of renal function.
The mechanism of action and the main pharmacological effects are the same for the entire class of ACE inhibitors. However, the choice of a specific drug from the ACE inhibitor group for the treatment of a specific patient may be important. ACE inhibitors are a heterogeneous group of drugs that differ from each other both in chemical structure, features of pharmacokinetics and pharmacodynamics, and in the presence of an evidence base for use for various indications. It is important to understand that although the ability of an ACE inhibitor to lower blood pressure and slow the progression of CHF are regarded as class-effects, many organ-protective effects of individual ACE inhibitors cannot be transferred from the standpoint of evidence-based medicine to the entire class of drugs.
ACE inhibitors differ in their chemical structure (the presence of a sulfhydryl group, etc.), metabolic characteristics (the presence of the effect of the first passage through the liver), the characteristics of excretion from the body (only by the kidneys or kidneys together with the liver), tissue specificity (the ability to block tissue RAAS) and duration actions (Table 3).
One of the most studied for a wide range of indications of ACE inhibitors is ramipril (Tritace ® ). The drug is characterized by high lipophilicity (superior to enalapril by almost 20 times), tissue specificity (superior to enalapril by 3-10 times, depending on tissue), a long half-life, which allows it to be used once a day. It is especially important to note that the evidence base for the use of ramipril in CVD, based on the results of RCTs with hard endpoints, is by far the largest among all ACE inhibitors.
The antihypertensive efficacy and safety of ramipril was evaluated in a large open-label study CARE conducted in a real clinical practice. The trial included 11100 patients with grade I-II hypertension, the effectiveness of treatment was assessed in 8261 patients. Ramipril was prescribed as monotherapy at a dose of 2.5 to 10 mg / day. After 8 weeks of treatment, there was a significant decrease in both SBP and DBP by an average of 13%, and this effect was also observed in the group of patients with isolated systolic hypertension (ISAG). The response rate to treatment (reaching target blood pressure below 140 and 90 mm Hg or decrease in DBP\u003e 10 mm Hg, or decrease in SBP\u003e 20 mm Hg with ISAH) was more than 85 in the systolic-diastolic hypertension group. %, and in the ISAG group more than 70%. The number of side effects during therapy, estimated in 11,100 patients, was low, and the cough rate did not exceed 3%.
Numerous studies have shown that ACE inhibitors are capable of causing regression of LVH and this effect is due not only to a decrease in blood pressure, but also to the blockade of the RAAS itself.
Meta-analyzes of RCTs that investigated the potential for different classes of antihypertensive drugs to induce regression of LVH also revealed the advantages of ACE inhibitors over other drugs.
The ability of ramipril to reduce the severity of LVH was studied in a double-blind, placebo-controlled RCT HYCAR ... In the course of the study, 115 patients with hypertension were prescribed either ramipril in doses of 1.25 mg / day. and 5 mg / day, or placebo. After 6 months, LV myocardial weight significantly increased in the placebo group and significantly decreased in the ramipril groups. A greater decrease was observed in the group with a dose of ramipril 5 mg / day. ... In an open multicenter RCT with a blind endpoint RACE in 193 patients with hypertension I-II stage. compared the effects of ramipril and atenolol on blood pressure levels and LV myocardial mass assessed by echocardiography. Ramipril was prescribed at a dose of 2.5 mg / day, atenolol at a dose of 50 mg / day. with the subsequent possibility of doubling the dose after 2 weeks. The duration of the test was 6 months. As a result, it was noted that both ramipril and atenolol significantly reduced both SBP and DBP, and equally. However, a significant decrease in the LV myocardial mass index was observed only in the ramipril group.
A large RCT has become an important milestone in the study of the possibilities of ACE inhibitors in the prevention of complications in high-risk patients HOPE (Heart Outcome Prevention Evaluation). The aim of the study was to assess the possibility of reducing morbidity and mortality from CVD in high-risk patients under the influence of two treatment strategies: ACE inhibitors ramipril and vitamin E. This double-blind, placebo-controlled study with a factorial design enrolled 9541 patients at high risk of cardiovascular complications due to age (\u003e 55 years), the presence of cardiac-vascular diseases or diabetes mellitus in combination with vascular disease or risk factors (hypertension, smoking, dyslipidemia). The peculiarities of the population of patients included in the study were the absence of LV dysfunction and CHF, a low mean baseline blood pressure (139 and 79 mm Hg), although almost half of those included in the study had AH, the use of other medicinal drugs that could affect treatment outcomes. So, 76% of patients received antiplatelet drugs (mainly acetylsalicylic acid (ASA)), 45% - calcium antagonists, 40% - β-adreno-blockers, 30% - lipid-lowering drugs, 15% - diuretics. In the course of the study, the frequency of the use of lipid-lowering drugs, β-blockers and diuretics increased, and the frequency of calcium antagonists decreased by 5%. The initially low values \u200b\u200bof blood pressure in the study population are explained precisely by the widespread use of antihypertensive drugs. Ramipril was prescribed starting at a dose of 2.5 mg / day, followed by titration up to 10 mg / day. The maximum dose by the end of the first year of the study was received by 82% of patients, and by the end of the study (4.5 years) - 65% of patients. The primary endpoint of the study was a combination of cardiovascular death, nonfatal MI, and nonfatal stroke.
The HOPE study was stopped early (six months earlier) due to the clear advantages of ramipril over vitamin E. The effectiveness of the latter did not differ from placebo. The rate of reaching the primary endpoint in the ramipril group was 14% compared with 17.8% in the placebo group, which corresponds to a 22% reduction in the relative risk (p<0,001). Относительный риск развития отдельных компонентов первичной конечной точки также снизился: инсульта на 32%, ИМ на 20%, сердечно-сосудистой смерти на 26%. Применение рамиприла обеспечило также достоверное снижение риска развития ХСН (на 23%) и проведения процедур реваскуляризации (на 15%). Важнейшим результатом исследования НОРЕ стало снижение под влиянием рамиприла общей смертности на 16% (р=0,005), причем кривые Капла-на-Майе-ра разошлись уже к первому году и продолжали расходиться до конца исследования.
The results of the study did not depend on the use of other drugs and were significant for various subgroups (with diabetes, hypertension, previous vascular lesions, men and women).
With ramipril, the incidence of new cases of diabetes was 33% lower than with placebo.
An important finding from the HOPE study was that the decline in endpoint occurrence was much more pronounced than expected from a BP decline. That is, the protective effects of ramipril clearly extended beyond its antihypertensive action. This made it possible to assume that ramipril actively influenced the processes of vascular remodeling and atherogenesis.
The ability of an ACE inhibitor to inhibit the development of atherosclerosis has been demonstrated in animals. However, human studies have produced conflicting results. Among all ACE inhibitors tested for the possibility of inhibition of atherogenesis in vivo, the largest evidence base has been accumulated for ramipril and perindopril. As part of the test NORE under investigation SECURE , which evaluated the ability of ramipril to slow the progression of atherosclerosis in 753 patients. With the use of a high dose of ramipril (10 mg / day), there was a 37% slowdown in the progression of atherosclerosis in the carotid artery compared with placebo, assessed by an increase in the thickness of the intima / media complex (IMC). In the low-dose ramipril group (2.5 mg / day), the IMC thickness was also smaller than in the placebo group, but the differences were not significant. Thus, the antiatherogenic effect of ramipril can be considered proven, but it should be considered as dose-dependent.
The anti-atherogenic effect of ramipril, identified in the SECURE study, apparently explains in many ways the drug's efficacy in secondary prevention of CVD, convincingly demonstrated in the HOPE study.
The continuation of the NORE study was the project NORE-TOO to assess whether ramipril continues to be able to reduce adverse cardiovascular events and new cases of diabetes over time in high-risk patients. The study included 4528 patients from the HOPE study who either continued to take ramipril 10 mg / day. in the open mode, or switched to ramipril after taking a placebo. By the end of the follow-up period (2.6 years), there was a further significant decrease in the relative risk of the primary endpoint by 17%, MI by 19%, revascularization procedures by 16%, and new cases of diabetes by 34%. Reductions in the relative risk of adverse events have been observed in various patient subgroups, including low, medium, and high risk subgroups. Thus, it has been proven that the protective effects of ramipril not only persist over time, but their severity is significantly higher than was shown in the HOPE study.
The use of ramipril for MI complicated by the development of heart failure has been studied in a large, double-blind, placebo-controlled RCT AIRE ... The trial included 2006 patients with confirmed MI and symptoms of heart failure. Ramipril was prescribed at a dose of 5 mg / day, starting from 3-10 days of illness, followed by titration up to 10 mg / day. within 2 days. The primary endpoint was overall mortality, the secondary endpoint was adverse cardiovascular events (death, re-heart attack, stroke, progression of heart failure). The duration of the study was 15 months on average. (minimum 6 months). 59% of patients in the ramipril group underwent thrombolysis, 77% took ASA, 25% - β-blockers, 56% - ni-waste. The use of ramipril provided a significant reduction in overall mortality by 27%, which became apparent after 30 days of treatment. The relative risk of secondary endpoints was significantly reduced by 19%. At the same time, the survival curves continued to diverge throughout the study (up to 30 months). The effect of ramipril persisted in various subgroups of patients (men and women, with and without hypertension, and others). The drug withdrawal rate was not significantly different from the placebo withdrawal rate.
Continuation AIRE there was a study AIREX , the purpose of which was to evaluate the effectiveness of long-term (5 years) therapy with ramipril in patients with MI with symptoms of heart failure. The trial included 603 patients from the AIRE study who continued to receive either ramipril or placebo. The duration of treatment was 59 months on average. (minimum 42 months). As a result, by the 59th month, the absolute survival rate was 11.4% higher in the ramipril group, which corresponds to a significant decrease in the relative risk of death by 36%. The average increase in life expectancy in the ramipril group was 1.45 g. As a result, not only was the high efficacy of the drug in this group of patients and its preservation over time confirmed again. It was also concluded that "treatment with ramipril 5 mg twice daily after acute MI, once started, should continue indefinitely."
The beneficial effect of ramipril on the survival of elderly patients with myocardial infarction was shown in a Canadian retrospective study, which included 7512 patients over 65 years old who received various ACE inhibitors after discharge from the hospital. As a result, ramipril significantly surpassed enalapril, fosinopril, captopril, quinapril and lisinopril in terms of its effect on survival during the first year.
Interesting comparative data were obtained when analyzing the outcomes of patients included in the registry MITRA PLUS ... Among 14608 patients with ST-segment elevation MI, 4.7% received ramipril, 39.0% - other ACE inhibitors, 56.3% - did not receive an ACE inhibitor. Compared with the absence of ACE inhibitors and, which is especially important, compared with other ACE inhibitors, treatment with ramipril provided significantly lower rates of hospital mortality and the incidence of adverse cardiovascular and cerebral events. However, there were no differences in the incidence of heart failure between ACE inhibitors.
Interesting data from a double-blind, placebo-controlled study DIAB-HYCAR , in which the effect of low doses of ramipril (1.25 mg / day) on the incidence of cardiovascular and renal complications in 4912 patients with type 2 diabetes and nephropathy manifested by microalbuminuria or proteinuria was evaluated. The use of the drug at such a low dose contributed to a slight decrease in blood pressure and a decrease in protein excretion in the urine, but did not lead to a significant decrease in either cardiovascular or renal endpoints. This result once again emphasizes that the beneficial effects of ramipril are realized when the appropriate doses are used - 10 mg / day.
The largest comparative RCT recently completed ONTARGET , which compared the possibilities of preventing complications in patients with CVD or diabetes mellitus without heart failure using three regimens of therapy: ACE inhibitors, ARBs, and a combination of ACE inhibitors + ARBs. The study included 25620 patients with coronary artery disease, peripheral vascular disease, cerebrovascular disease or diabetes mellitus. At baseline, 89% of patients had CVD, 69% had hypertension, and 38% had diabetes. When included in the study, 80.9% of patients took antiplatelet agents, 61.6% - statins, 56.9% - β - adrenergic blockers, 28.0% - diuretics. Patients were randomized into three groups: taking ramipril at a dose of 10 mg / day. (n \u003d 8502) taking telmisartan 80 mg / day. (n \u003d 8542) and taking a combination of ramipril with telmisartan (n \u003d 8502). The observation period was 56 months.
As a result, 16.5% of patients in the ramipril group, 16.7% in the telmisartan group and 16.3% in the combination treatment group achieved the primary combined endpoint, which included mortality from complications of CVD, MI, stroke, or hospitalization for heart failure. That is, there was no difference between ramipril monotherapy, telmisartan monotherapy, and combination therapy with both drugs. The incidence of individual adverse outcomes included in the composite indicator and overall mortality did not differ significantly either. At the same time, deterioration of renal function was more often observed in the combination therapy group: the relative risk of developing chronic renal failure was 1.33 (p<0,001) .
Thus, this largest comparative study did not reveal the advantages of using ARBs over traditional therapy with ACE inhibitors in patients with CVD and diabetes mellitus, with the exception of a slightly lower incidence of angioedema. In fact, telmisartan at a dose of 80 mg / day. provided 94% of the effectiveness of ramipril at the 10 mg / day dose established in the HOPE study. These data are consistent with the results of the RCT VALIANT, in which the effect of valsartan also did not exceed the effect of captopril.
All this allowed J.McMurray in an editorial New England Journal of Medicineto express the opinion that since ARBs are not superior to traditional ACE inhibitors in terms of effectiveness, but are significantly more expensive, their area of \u200b\u200bapplication mainly comes down to cases of intolerance to ACE inhibitors due to cough.
The results of the ONTARGET study are of great scientific importance, not only in practical terms. They once again force us to pay attention to the alleged role of bradykinin in ensuring the clinical efficacy of drugs that block RAAS. And although ACE inhibitors do not completely block the formation of Ang II, unlike ARBs, they reduce the degradation of bradykinin to inactive metabolites.
Thus, the available RCT results with ramipril indicate that the drug provides a positive effect on endpoints, including overall mortality, in various CVDs. In fact, this makes it possible to provide organoprotection at various stages of the cardiovascular (including the hypertensive cascade) continuum, ranging from exposure to risk factors (primarily hypertension and diabetes) and ending with terminal organ damage (CHF). At the same time, it is necessary to emphasize the importance of choosing the correct dosage for the drug and the need for long-term, often lifelong treatment.



Literature
1. Ezzati M, Hoorn SV, Rodgers A, et al. 2003. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet 362: 271-80.
2. Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J. 1991 Apr; 121 (4 Pt 1): 1244-63.
3. Victor J. Dzau, Elliott M. Antman, Henry R. Black et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006 Dec 19; 114 (25): 2850-70.
4. V. I. Podzolkov, V. A. Bulatov. Myocardium. Nephron. A look through the prism of the evolution of arterial hypertension. RMJ 2008, 16 (11): 1517-1523.
5. Morgan L, Broughton PF, Kalsheker N. Angiotensinogen: molecular biology, biochemistry and physiology. Int J Biochem Cell Biol. 1996; 28: 1211-22.
6. Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003; 24: 261-71.
7. Reudelhuber TL. The renin-angiotensin system: peptides and enzymes beyond angiotensin II. Curr Opin Nephrol Hypertens. 2005; 14: 155-59.
8. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000 Sep 1; 87 (5): E1-9.
9. Tallant EA, Ferrario CM, Gallagher PE. Angiotensin- (1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 2005; 289: H1560-H1566.
10. Stanton A. Therapeutic potential of renin inhibitors in the management of cardiovascular disorders. Am J Cardiovasc Drugs. 2003; 3: 389-94.
11. Ichihara A, Hayashi M, Kaneshiro Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004; 114: 1128-35.
12. Phillips MI. Tissue renin-angiotensin systems. In: Izzo JL, Black HR, ed. Hypertension Primer: The Essentials of High Blood Pressure. 2nd ed. Baltimore, MD: Lippincott William & Wilkins; 1999: 23-24.
13. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006; 86: 747-803.
14. Lopez-Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J. 2004 Aug; 25 (16): 1454-70.
15. Kaplan NM. The CARE Study: a postmarketing evaluation of ramipril in 11,100 patients. The Clinical Altace Real-World Efficacy (CARE) Investigators. Clin Ther. 1996 Jul-Aug; 18 (4): 658-70.
16. Dahlof B, Pennert K, Hansson L. Reversal of left ventricular hypertrophy in hypertensive patients. A metaanalysis of 109 treatment studies. Am J Hypertens. 1992 Feb; 5 (2): 95-110.
17. Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double-blind studies. JAMA. 1996 May 15; 275 (19): 1507-13.
18. Lievre M, Gueret P, Gayet C, et al. Remission of left ventricular hypertrophy with ramipril independently of blood pressure changes: the HYCAR study (cardiac hypertrophy and ramipril)] Arch Mal Coeur Vaiss. 1995 Feb; 88 Spec # 2: 35-42.
19. Agabiti-Rosei E, Ambrosioni E, Dal Palu C, Muiesan ML, Zanchetti A. ACE inhibitor ramipril is more effective than the beta-blocker atenolol in reducing left ventricular mass in hypertension. Results of the RACE (ramipril cardioprotective evaluation) study on behalf of the RACE study group. J Hypertens. 1995 Nov; 13 (11): 1325-34.
20. The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on death from cardiovascular causes, myocardial infarction and stroke in high-risk patients.New Engl J Med 2000; 342: 145-153.
21. Pitt B. Potential role of angiotensin-converting enzyme inhibitors in the treatment of atherosclerosis. Eur Heart J 1995; 16: 49-54.
22. Schoelkens BA, Landgraf W. ACE inhibition and atherosclerosis. Can J Physiol Pharmacol 2002; 80: 354-9.
23. Lonn E, Yusuf S, Dzavik V, et al. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation. 2001 Feb 20; 103 (7): 919-25.
24. Bosch J, Lonn E, Pogue J, Arnold JM, Dagenais GR, Yusuf S; HOPE / HOPE-TOO Study Investigators. Long-term effects of ramipril on cardiovascular events and on diabetes: results of the HOPE study extension. Circulation. 2005 Aug 30; 112 (9): 1339-46.
25. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993 Oct 2; 342 (8875): 821-8.
26. Hall AS, Murray GD, Ball SG. Follow-up study of patients randomly allocated ramipril or placebo for heart failure after acute myocardial infarction: AIRE Extension (AIREX) Study. Acute Infarction Ramipril Efficacy. Lancet. 1997 May 24; 349 (9064): 1493-7.
27. Pilote L, Abrahamowicz M, Rodrigues E, Eisenberg MJ, Rahme E. Mortality rates in elderly patients who take different angiotensin-converting enzyme inhibitors after acute myocardial infarction: a class effect? Ann Intern Med. 2004 Jul 20; 141 (2): 102-12.
28. Wienbergen H, Schiele R, Gitt AK, et al. Impact of ramipril versus other angiotensin-converting enzyme inhibitors on outcome of unselected patients with ST-elevation acute myocardial infarction. Am J Cardiol. 2002 Nov 15; 90 (10): 1045-9.
29. Michel Marre, Michel Lievre, Gilles Chatellier, et al. on behalf of the DIABHYCAR Study Investigators. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomized, double blind, placebo controlled trial (the DIAB-HYCAR study). BMJ 2004; 328; 495.
30. The ONTARGET Investigators. Telmisartan, ramipril or both in patients at a high risk for vascular events. N Eng J Med 2008; 358: 1547-1559
31. Pfeffer M, McMurray J, Velazquez E, et al. Valsartan, captopril or both in myocardial infarction complicated by heart failure, left ventricle dysfunction. N Eng J Med 2003; 349: 1893-1906
32. McMurray J. ACE-inhibitors in cardiovascular disease - unbeatable? N Eng J Med 2008; 358: 1615-1616
Read also ...
- Cefazolin: how to dilute novocaine for injection for children and adults?
- Motherwort - treatment, recipes, instructions
- Bromhexin tablets and syrup for children: instructions for use and what it is for, price, reviews Bromhexin instructions for use tablets are taken
- Interferon instructions for use in tablets



















